Long Chain Alkylamines
Long chain alkylamines such as TBA (Tributylamine) and TPA (Tripropylamine) were
analysed by ion-pair method using RI detector. However, this method is not so
sensitive. Using IC
YK-421 with nitric acid aqueous solution/
acetonitrile eluent and a conductivity detector, highly sensitive analysis of
long chain alkylamines can be realized.The effect of acetonitrile concentration
on elution volume of alkylamines are shown. When the acetonitrile concentration
is lower than 50%, the components which have shorter chain elute faster. And,
to the contrary, when the acetonitrile concentration if higher than 50%, the components
which have longer chain elute faster. The reason of the difference can be explained
by the the difference of the separation mode. When acetonitrile concentration
is lower than 50%, the separation mode is hydrohorbic interaction, and, when acetonitrile
concentration is higher than 50% the separation mode is ion exchange.
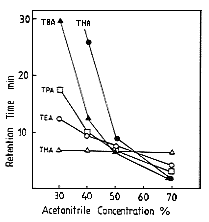
Sample :
Alkylamines
THA, Trihexylamine
TBA, Tributylamine
TPA, Tripropylamine
TEA, Triethylamine
TMA, Trimethylamine
Column : Shodex IC YK-421 (4.6mmID*125mm)
Eluent : 3mM Nitric acid aq./CH3CN
Flow rate : 1.0mL/min
Detector : Non-suppressed coductivity
Column temp. : 40deg-C
