Effect of pH on Elution Pattern (DEAE-825)
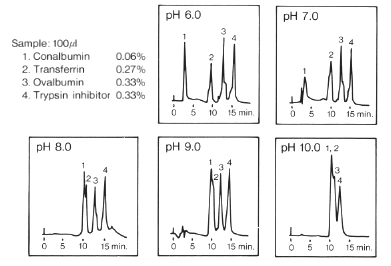
Sample:
1. Conalbumin
2. Transferrin
3. Ovalbumin
4. Trypsin inhibitor
Column : Shodex IEC DEAE-825 (8.0mmID*75mm)
Eluent : (A); 20mM Piperazine-HCl buffer(pH6.0)
20mM Tris-Trispropanol-HCl buffer(pH7.0)
20mM Tris-HCl buffer(pH8.0)
20mM Ethanolamine-HCl(pH9.0)
20mM 1,3-Diaminopropane-HCl buffer(pH10.0)
(B); (A) + 0.5M NaCl
Linear gradient: 0min to 20min, (A) to (B)
Flow rate : 1.0mL/min