Measurement of Molecular Weight Distribution of Poly(Allylamine) Hydrochloride
Poly(allylamine) is one of the cationic polymer. In SEC analysis of cationic polymer,
the selection of eluent which prevents ionic interaction and hydrophobic interaction
between a sample and gel makes measurement successful. In order to control the
ionic interaction between a sample and gel, salt should beadded to the eluent.
But if the salt containing multivalent anion such as phosphate or sulfate is used,
a sample may not dissolve or a sample may precipitate after the dissolution. On
the other hand, if the salt containing one valent anion such as nitrate and chloride
is used, precipitation of a sample will not take place. Here, the influence to
the chromatogram of the salt concentration in the eluent was investigated under
the various concentration of sodium nitrate in the eluent. In order to reduce
hydrophobic interaction between a sample and gel, 0.5M acetic acid was added to
the eluent. Comparison of chromatograms of poly(allylamine) measured under the
various concentration of sodium nitrate in the eluent is shown in Fig.1. In the
case of 0.02M sodium nitrate in the eluent, the constituent of high molecular
weight of poly(allylamine) is adsorbed to the gel material and is not eluted from
a column. When the salt concentration of sodium nitrate is more than 0.1M, the
adsorption of a sample is suppressed and a right chromatogram is obtained. The
relation between the molecular weight of poly(allylamine) and elution volume with
SEC/MALS is shown in Fig.2. Under the 0.1M - 0.2M of sodium nitrate in the eluent,
poly(allylamine) is eluted in the molecular weight order. The relation between
the RMS radius (size of the molecule in the solution) of poly(allylamine) and
elution volume with SEC/MALS is shown in Fig.3. When the concentration of sodium
nitrate is low, molecule of the polymer spreads out and RMS radius becomes large,
as the result, poly(allylamine) is eluted early. When the salt concentration is
0.1-0.2M, we can see the influence of the diffusion of molecule is decreasing
according to the salt concentration, and separation is performed in the molecular
size order. From the above result, in the measurement of poly(allylamine) with
SB-806M HQ, the eluent of 0.5M acetic acid + 0.1M - 0.2M
sodium nitrate is sutable for this analysis.
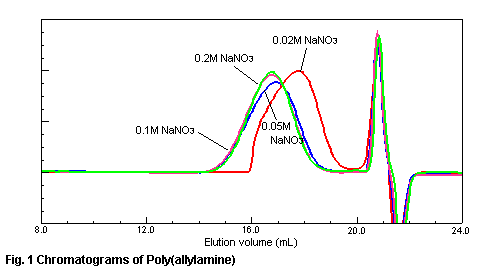
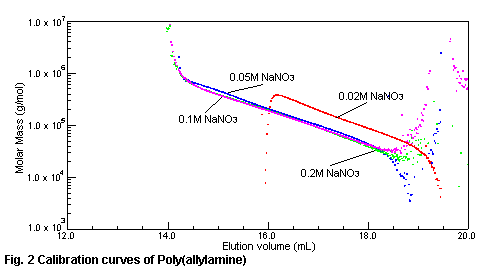
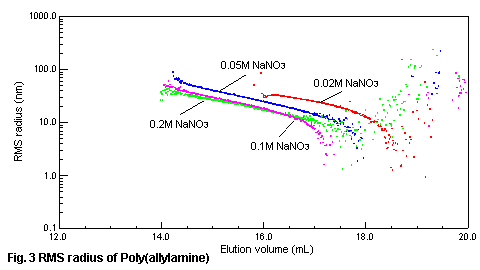
Sample : 0.2%
Poly(allylamine), 100micro-L
Columns : Shodex OHpak SB-806M HQ (8.0mmID*300mm) x 2
Eluent : 0.5M CH3COOH + nM NaNO3 aq.
Flow rate : 1.0mL/min
Detector : Shodex RI, MALS(Multi-Angle Laser Light Scattering Detector)
Column temp. : 40deg-C


