Hydrogen Peroxide
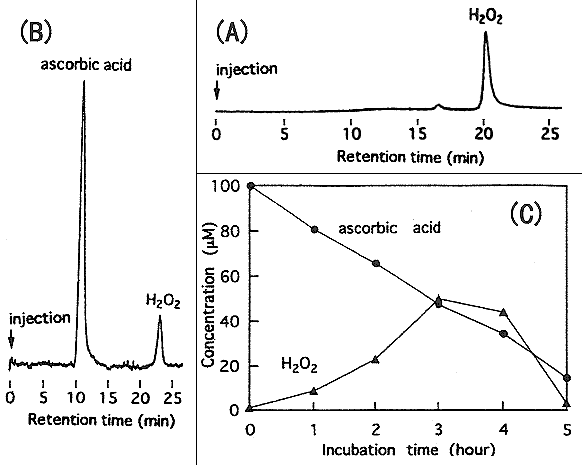
(A) shows the detection of hyrogen peroxide alone. The peak of hydrogen peroxide can be seen at 21.2 minutes. The small peak at 16.8 minutes is considered to be an impurity in hydrogen peroxide.
(B) shows the chromatogram of simultaneous analysis of ascorbic acid and hydrogen peroxide.
(C) shows the autoxidation of ascorbic acid. The quantitaion was done using HPLC detection. (B) is the chromatogram at 3 hours in (C).
Sample :
Hydrogen peroxide
Ascorbic acid
Column : Shodex SUGAR KS-801 (8.0mmID*300mm) Eluent : H2O Reagent : 10mM Na2SO4 + 10 micro-M EDTA-2Na aq. Flow rate : (Eluent); 0.6mL/min, (Reagent); 0.15mL/min Detector : ECD(Electrode; Pt, 500mV vs Ag/AgCl) Column temp. : 40deg-C
Takahashi et al., Analytycal Sciences, 15 (1999) 481