Transition Metal Ions and Rare Earth Metal Ions
| Guard column |
Analitical column |
Comments | |
|---|---|---|---|
| Transition Metal Ions |
IC T-G
|
IC T-521
|
All piping including injector should be non-metal. |
| Rare Earth Ions |
IC R-G
|
IC R-621
|
Stainless steel piping can be used. Gradient system is necessary. Acid concentration of sample should be lower than 1N. |
Transition metal ions are separated using two columns of IC Y-521.
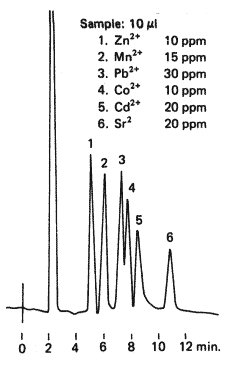
Sample :
1. Zn2+
2. Mn2+
3. Pb2+
4. Co2+
5. Cd2+
6. Sr2+
Columns : Shodex IC Y-521 (4.6mmID*150mm) x 2 Eluent : 4mM Tartaric acid + 2mM Ethylenediamine aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity, Polarity(-) Column temp. : 40deg-C
Transition metal ions were analyzed using IC T-521. An oxalic acid/tartaric acid eluent is used for the analysis of transition metal ions. Each transition metal ion generates complex ions with the organic acids in the eluent at a different rate. The easier the transition metal ions generate the complex ions, the faster they elute. The sample should be dissolved in a 40mM HCl aqueous solution.
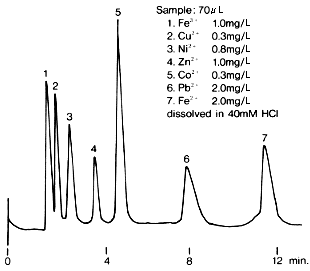
Sample : 70micro-L
1. Fe3+
2. Cu2+
3. Ni2+
4. Zn2+
5. Co2+
6. Pb2+
7. Fe2+
Column : Shodex IC T-521 (4.6mm*150mm) Eluent : 6mM Oxalic acid + 3mM Citric acid + 0.1mM NaN3 + 0.1mM NaHSO3(pH4.1 adjusted by KOH) Reagent : 0.2mM *PAR + 3M NH4OH + 1M CH3COOH (Mixing coil(0.5mmID*1m)) Flow rate : (Eluent); 1.0mL/min, (Reagent); 1.0mL/min Detector : VIS(530nm) Column temp. : 40deg-C
*PAR : 4-(2-Pyridylazo)resorcinol
With 6mM Tartaric asid and 4mM oxalic asid aqueous solution as the eluent, transision metals, such as nickel, zinc, and cobalt can be analyzed using IC YS-50.
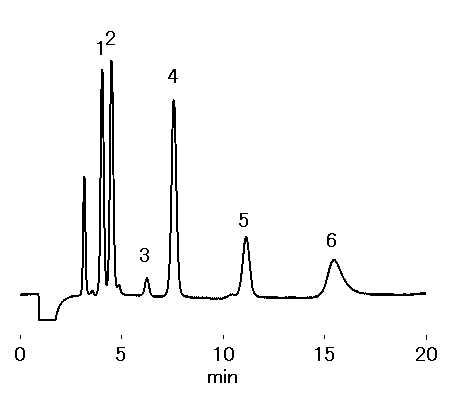
Sample : 20micro-L
1. Zn2+ 10mg/L
2. Co2+ 10mg/L
3. Fe2+ 10mg/L
4. Mn2+ 10mg/L
5. Cd2+ 10mg/L
6. Pb2+ 30mg/L
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 6mM Tartaric acid + 4mM Oxalic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Transition metal ions were analyzed using IC T-521. Fe3+, Zn2+, Fe2+ and Mn2+ are contained in red wine. It takes about 30 minutes to separate Mn2+.
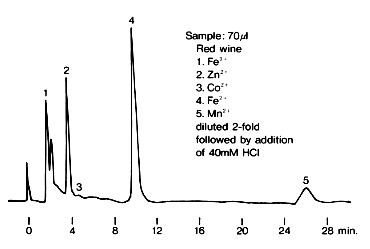
Sample : Red wine
1. Fe3+
2. Zn2+
3. Co2+
4. Fe2+
5. Mn2+
Column : Shodex IC T-521 (4.6mmID*150mm) Eluent : 6mM Oxalic acid + 3mM Citric acid + 0.1mM NaN3 + 0.1mM NaHSO3(pH4.1 adjusted by KOH) Reagent : 0.2mM *PAR + 3M NH4OH + 1M CH3COOH (Mixing coil(0.5mmID*1m)) Flow rate : (Eluent); 1.0mL/min, (Reagent); 1.0mL/min Detector : VIS(530nm) Column temp. : 40deg-C
*PAR : 4-(2-Pyridylazo)resorcinol
Transition metal ions in tap water were analyzed using IC T-521. Fe3+ and Zn2+ are contained in tap water. HCl is added to the sample to ionize Fe to Fe3+ completely. (The final HCl concentration is 40mM)
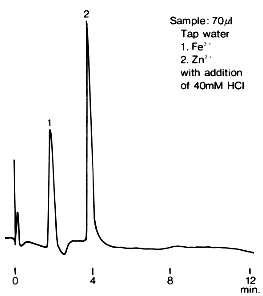
Sample : Tap water
1. Fe3+
2. Zn2+
Column : Shodex IC T-521 (4.6mm*150mm) Eluent : 6mM Oxalic acid + 3mM Citric acid + 0.1mM NaN3 + 0.1mM NaHSO3(pH4.1 adjusted by KOH) Reagent : 0.2mM *PAR + 3M NH4OH + 1M CH3COOH (Mixing coil(0.5mmID*1m)) Flow rate : (Eluent); 1.0mL/min, (Reagent); 1.0mL/min Detector : VIS(530nm) Column temp. : 40deg-C
*PAR : 4-(2-Pyridylazo)resorcinol
Highly sensitive analysis of cobalt ion is possible using IC T-521 and a chemiluminescence detector (CLD). When 500 micro-L of sample is directly injected, ng/L(=ppt) level detection is possible. Reagents should be prepared just before they are used. And, they should be kept cooling with ice water in a dark place.
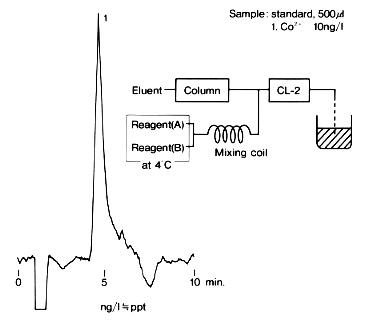
Sample : Co2+
Column : Shodex IC T-521 (4.6mmID*150mm) Eluent : 6mM Oxalic acid + 3mM Citric acid + 0.1mM NaN3(pH4.1) Reagent : (A); 1mM Luminol in 0.1M Borate buffer(pH11.0), (B); 1mM H2O2 Flow rate : (Eluent); 1.0mL/min, (Reagent A); 0.5mL/min, (Reagent A); 1.5mL/min Detector : CLD Column temp. : 40deg-C
Rare earth metals ions were analyzed using IC R-621. Rare earth metals ions can be analyzed according to the difference of the speed in generating complex ions with alpha-hydroxyisobutyric acid. Baseline separation of fifteen rare earth metal ions is possible in 30 minutes.
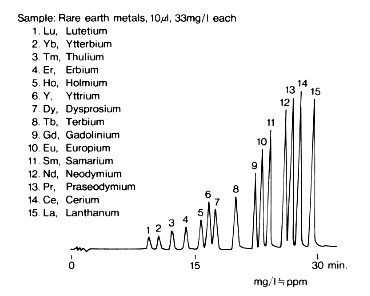
| Sample : 1. Lu, Lutetium 2. Yb, Ytterbium 3. Tm, Thulium 4. Er, Erbium 5. Ho, Holmium 6. Y, Yttrium 7. Dy, Dysprosium 8. Tb, Terbium 9. Gd, Gadolinium 10. Eu, Europium 11. Sm, Samarium 12. Nd, Neodymium 13. Pr, Praseodymium 14. Ce, Cerium 15. La, Lanthanum |
Columns : Shodex IC R-G (4.6mmID*10mm) + R-621 (6.0mmID*50mm)
Eluent : (A); 0.1M alpha-Hydroxyisobutyric acid(pH4.0,adjusted by NaOH)
(B); 1M alpha-Hydroxyisobutyric acid(pH4.0,adjusted by NaOH)
Step gradient: 0min to 15min, 100% (A)
15min to 25min, 20% (B)
after 25min, 100% (B)
Reagent : 0.005(W/V)% Arsenazo III/0.1M Formate(pH3.5)
Flow rate : (Eluent); 1.0mL/min, (Reagent); 1.0mL/min
Detector : VIS(655nm)
Column temp. : 40deg-C
Rare earth metal ions in yttrium ore were analyzed using IC R-621. Yttrium ore is heated and dissolved in 1% HCl solution and diluted to 0.2% with purified water.
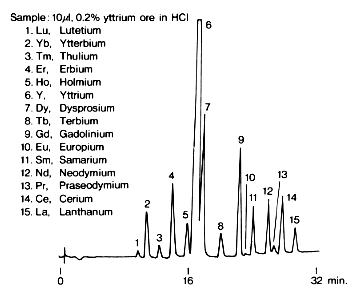
| Sample : 1. Lu, Lutetium 2. Yb, Ytterbium 3. Tm, Thulium 4. Er, Erbium 5. Ho, Holmium 6. Y, Yttrium 7. Dy, Dysprosium 8. Tb, Terbium 9. Gd, Gadolinium 10. Eu, Europium 11. Sm, Samarium 12. Nd, Neodymium 13. Pr, Praseodymium 14. Ce, Cerium 15. La, Lanthanum |
Columns : Shodex IC R-G (4.6mmID*10mm) + R-621 (6.0mmID*50mm)
Eluent : (A): 0.1M alpha-Hydroxyisobutyric acid(pH4.0,adjusted by NaOH)
(B): 1M alpha-Hydroxyisobutyric acid(pH4.0,adjusted by NaOH)
Step gradient: 0min to 15min, 100% (A)
15min to 25min, 20% (B)
after 25min, 100% (B)
Reagent : 0.005(W/V)% Arsenazo III/0.1M Formate(pH3.5)
Flow rate : (Eluent); 1.0mL/min, (Reagent); 1.0mL/min
Detector : VIS(655nm)
Column temp. : 40deg-C
Rare earth metal ions in Nd-Dy magnetic alloy were analyzed using IC R-621. 0.5g of the alloy is heated and dissolved in a mixture of 10mL of purified water and 5mL of HNO3, and then diluted to 0.2% with purified water.
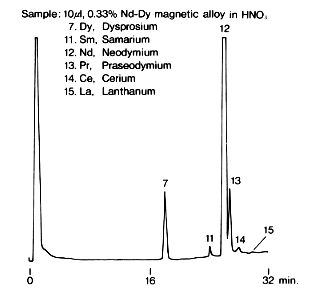
Sample :
7. Dy, Dysprosium
11. Sm, Samarium
12. Nd, Neodymium
13. Pr, Praseodymium
14. Ce, Cerium
15. La, Lanthanum
Columns : Shodex IC R-G (4.6mmID*10mm) + R-621 (6.0mmID*50mm)
Eluent : (A): 0.1M alpha-Hydroxyisobutyric acid(pH4.0,adjusted by NaOH)
(B): 1M alpha-Hydroxyisobutyric acid(pH4.0,adjusted by NaOH)
Step gradient: 0min to 15min, 100% (A)
15min to 25min, 20% (B)
after 25min, 100% (B)
Reagent : 0.005(W/V)% Arsenazo III/0.1M Formate(pH3.5)
Flow rate : (Eluent); 1.0mL/min, (Reagent); 1.0mL/min
Detector : VIS(655nm)
Column temp. : 40deg-C
A published LC-ICP-MS speciation method for Cr(III) and Cr(VI) was carried using customized RSpak NN column*1. For this customized column, RSpak NN series gel was packed into 4.6 x 150 mm PEEK column housing instead of standard SUS housing. NN series packed gel is made from polyhydroxymethacrylate base modified with sulfo functional group. The main separation mode is reversed phase, but the addition of sulfo group provides ionic interaction effects. In this application, Cr(III) and Cr(VI) were analyzed according to the published method.
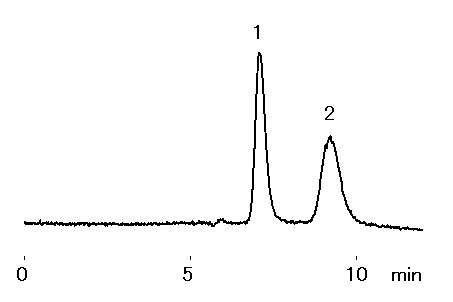
Sample : 5micro-g/L each, 25micro-L
1. Cr(III), Cr(NO3)3
2. Cr(VI), K2CrO4
Sample was first prepared by using 10mM nitric acid to make a 1 mg/L solution; and then it was diluted with mobile phase to the final concentration of 5micro-g/L.
Column : Shodex *1NN-414 4D(4.6mmID*150mm) PEEK housing type can be prepared as a customized order Eluent : 90mM (NH4)2SO4 + 10mM NH4NO3(pH3.0 adjusted by HNO3) Flow rate : 0.3mL/min Detector : ICP/MS, Cr at m/z=52 Column temp. : 40℃
Reference:
Harald Hagendorfer, Walter Goessler.
Separation of chromium(III) and chromium(VI) by ion chromatography and an inductively coupled plasma mass spectrometer as element-selective detector.
Talanta. 2008, vol.76, p.656-661.
