Effect of pH on Elution Pattern (DEAE-825)
When the buffer eluent is acidic, four types of proteins are separated using IEC DEAE-825 (a column for weak anion exchange chromatography) well. In opposition to this, when the eluent is alkaline, the separation of the proteins is poor. By increasing the pH value, the four peaks tend to converge into one peak. The reason for that is the ion exchange groups of the packing material cannot dissociate adequately when the pH value is high.
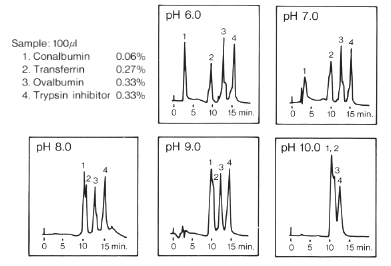
Sample:
1. Conalbumin
2. Transferrin
3. Ovalbumin
4. Trypsin inhibitor
Column : Shodex IEC DEAE-825 (8.0mmID*75mm)
Eluent : (A); 20mM Piperazine-HCl buffer(pH6.0)
20mM Tris-Trispropanol-HCl buffer(pH7.0)
20mM Tris-HCl buffer(pH8.0)
20mM Ethanolamine-HCl(pH9.0)
20mM 1,3-Diaminopropane-HCl buffer(pH10.0)
(B); (A) + 0.5M NaCl
Linear gradient: 0min to 20min, (A) to (B)
Flow rate : 1.0mL/min
