Cations(Simultaneous separation of monovalent and divalent cations: YS-50, YK-421)
(IC YS-50 Column)
1) Can apply to simultaneous analysis of monovalent and divalent cations with isocratic separation.
IC YS-50 enables simultaneous analysis of monovalent and divalent cations at a higher sensitivity than conventional column (IC YK-421). With about twice the theoretical plate number and about 1.3 times the Na+ / NH4+ separatability, the determination performance of NH4+ at a high concentration of Na+ has drastically improved (Na+ : NH4+ = 5,000 : 1 is possible).
2) Quantitative analysis of divalent cations are also improved.
Comparing with conventional column, the theoretical plate number becomes twice. Since peak shapes of divalent cations are improved, so quantitative analysis of divalent cations are also improved.
3) Various acids as the eluent can be used.
Not only methanesulfonic acid but sulforic acid, nitric acid, phospphoric acid, etc. can use it as the eluent.
4) Suitable for analysis of alkylamines.
Analysis of various alkylamines, such as Methylamine and Trimethylamine, is possible.
5) Can apply to analysis of transision metals.
With 6mM Tartaric asid and 4mM oxalic asid aqueous solution as the eluent, transision metals, such as nickel, zinc, and cobalt can be analyzed.
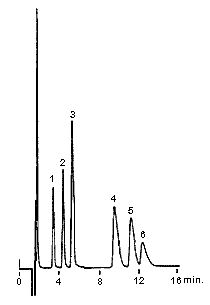
The six main cations can be separated using a newly developed column, IC YS-50. Comparing with the conventional column IC YK-421, the theoretical plate number of divalent cations becomes more than double and the separation of Na+ and NH4+ becomes about 1.2 times.
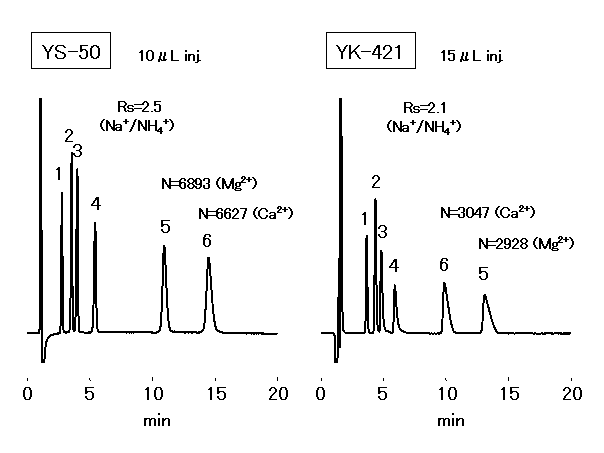
Sample :
1. Li+ 2ppm
2. Na+ 10ppm
3. NH4+ 10ppm
4. K+ 20ppm
5. Mg2+ 10ppm
6. Ca2+ 20ppm
Columns : Shodex IC YS-50 (4.6mmID*125mm) Shodex IC YK-421 (4.6mmID*125mm)
Eluent : 4mM Methanesulfonic acid aq. 5mM Tartaric acid + 1mM Dipicolinic acid
+ 1.5g/L Boric acid aq.
Flow rate : 1.0mL/min 1.0mL/min
Detector : Non-suppressed conductivity Non-suppressed conductivity
Column temp. : 40deg-C 40deg-C
Calibration curves for cations analyzed using IC YS-50 by non-suppessor method are shown. Each of which has excellent linearity with a correlation of 0.999 or above
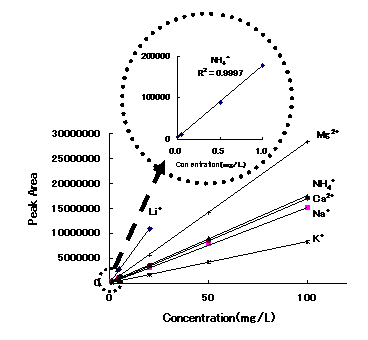
Sample : 10micro-L
|
Correlation Coefficient
1ppm to 100ppm |
|
| Li+ | 1.000 |
| Na+ | 0.9998 |
| NH4+ | 0.9997 |
| K+ | 1.0000 |
| Mg2+ | 1.0000 |
| Ca2+ | 1.0000 |
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
With about twice the theoretical plate number and about 1.3 times the Na+/NH4+ resolution, YS-50 can be applied to NH4+ analysis at a high concentration of Na+ (Na+:NH4+=5,000:1 is possible).
Please refet to Separation of Na+ and NH4+ (2) (Suppressor Method)
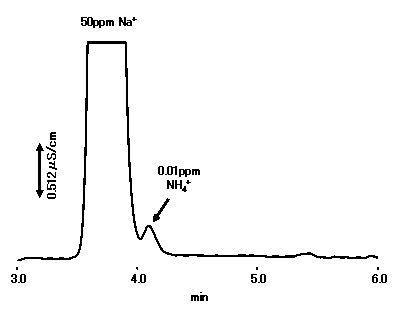
Sample : 10micro-L
Na+ 50ppm
NH4+ 0.01ppm
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Using IC YS-50, changing the concentrationt of methanesulfonic acid in the eluent enables to control cations separation and retetntion time. Although, retention time of cations will become short if concentration is high, the eluent conductivity (background level) becomes also high.
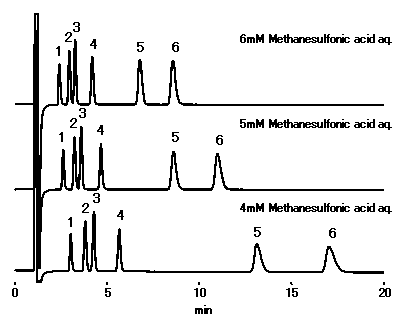
Sample : 10micro-L
1. Li+ 2ppm
2. Na+ 10ppm
3. NH4+ 10ppm
4. K+ 20ppm
5. Mg2+ 10ppm
6. Ca2+ 20ppm
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Although the optimal flow rate is 0.8 or less mL/min, there are few falls of theoretical plate number at 1.0 mL/min, and since ca2+ can be analyzed within 20 minutes, by general cation analysis using IC YS-50, We recommend to set flow rate below to 1.0mL/min.
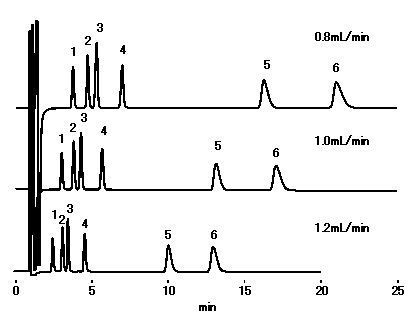
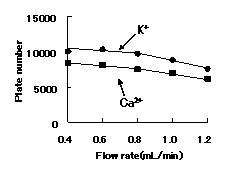
Sample : 10micro-L
1. Li+ 2ppm
2. Na+ 10ppm
3. NH4+ 10ppm
4. K+ 20ppm
5. Mg2+ 10ppm
6. Ca2+ 20ppm
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Detector : Non-suppressed conductivity Column temp. : 40deg-C
Ethylenediamine (0.1% v/v of 50 mg/mL) is added to water samples to prevent HCl production from the reaction between residual chloride and chlorous acid. Ion chromatography (cation) column, IC YS-50 was used to separate mono- and di-valent cations in the presence of ethylenediamine. 10% ACN was added to the mobile phase to shorten ethylenediamine retention. Addition of crown ether can improve the separation of Na+ and NH4+ , but this will increase the ethylenediamine retention.
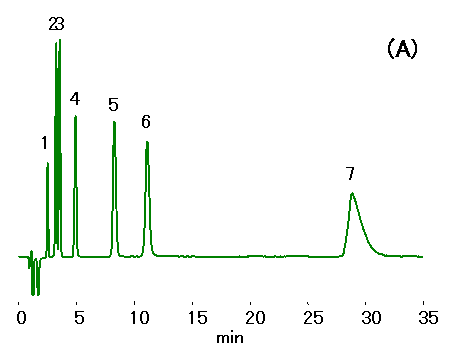
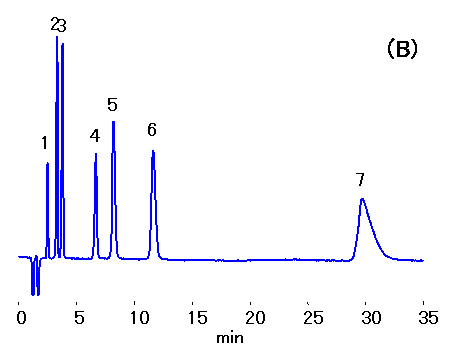
Sample : Cation std.,10micro-L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. K+ 20mg/L
5. Mg2+ 10mg/L
6. Ca2+ 20mg/L
7. Ethylenediamine, EDA 50mg/L
Column : Shodex IC YS-50 (4.6mmID*125mm)
Eluent : (A); 4mM Nitric acid aq./CH3CN=90/10
(B); 4mM Nitric acid + 1.5mM 18-Crown 6-ether aq./CH3CN=90/10
Flow rate : 1.0mL/min
Detector : Non-suppressed conductivity
Column temp. : 40deg-C
The effect of temperature for IC YS-50 was investigated. This shows no significant influence of temperature on elution.
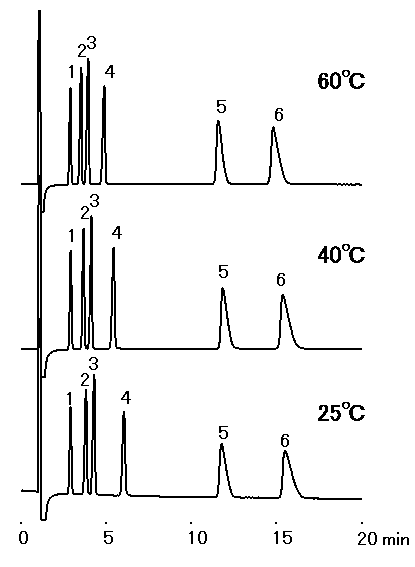
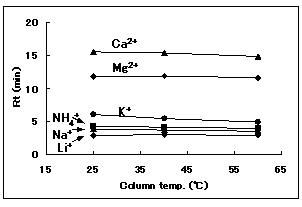
Sample : 10micro-L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. K+ 20mg/L
5. Mg2+ 10mg/L
6. Ca2+ 20mg/L
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity
IC YS-50 shows good efficiency until 50ppm of cation(K+) concentration. Recommended sample loading is less than 10micro-L per column.
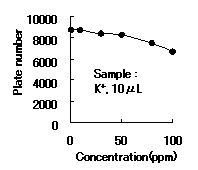
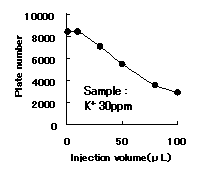
Sample : K+
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
IC YS-50 can use the eluent adding acetonitrile (up to 50%). Adding acetonitrile can weaken hydrophobic interaction, and therefore quicken the elution of highly hydrophobic cations.
Note: Not every organic solvent can be used. (Methanol is unusable.)
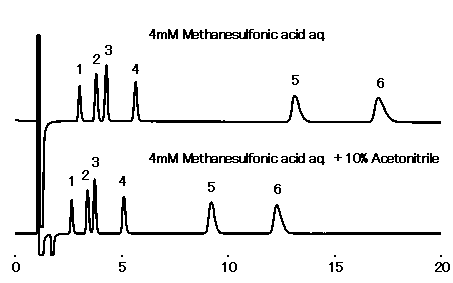
Sample : 10micro-L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. K+ 20mg/L
5. Mg2+ 10mg/L
6. Ca2+ 20mg/L
Column : Shodex IC YS-50 (4.6mmID*125mm) Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Acid selection depends on the sample type, instrument, and analysis conditions. Cation standards analyzed using IC YS-50 with 4 mM methanesulfonic acid, sulfuric acid, nitric acid, and phosphoric acid as the eluent are shouwn.
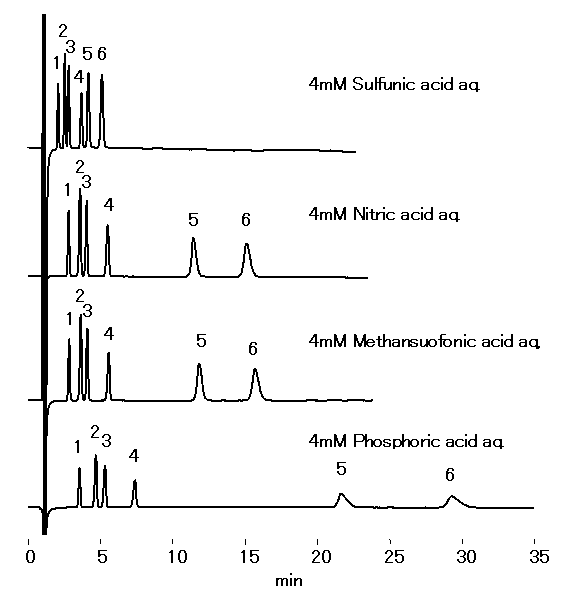
Sample : 10micro-L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. K+ 20mg/L
5. Mg2+ 10mg/L
6. Ca2+ 20mg/L
|
Eluent
|
Conductivity
(micro-S/cm) |
| 4mM Sulfuric acid aq. |
2906
|
| 4mM Nitric acid aq. |
1926
|
| 4mM Methanesulfonic acid aq. |
1733
|
| 4mM Phosphoric acid aq. |
1237
|
Column : Shodex IC YS-50 (4.6mmID*125mm) Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
IC YS-50 can be applied to suppressor method. With about twice the theoretical plate number and about 1.3 times the Na+/NH4+ resolution, YS-50 can be applied to NH4+ analysis at a high concentration of Na+ (Na+:NH4+=5,000:1 is possible).
Please refer to Separation of Na+ and NH4+ (1) (Non-suppressor Method)
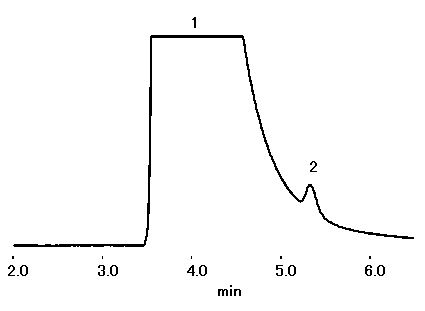
Sample : 10micro-L
1. Na+ 50ppm
2. NH4+ 0.01ppm
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Suppressed conductivity Column temp. : 40deg-C
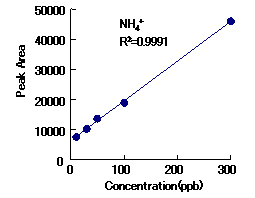
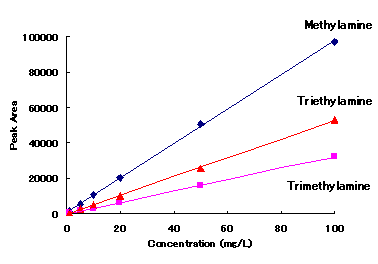
Cation standards and alkylamines can be analyzed simultaneously with IC YS-50.
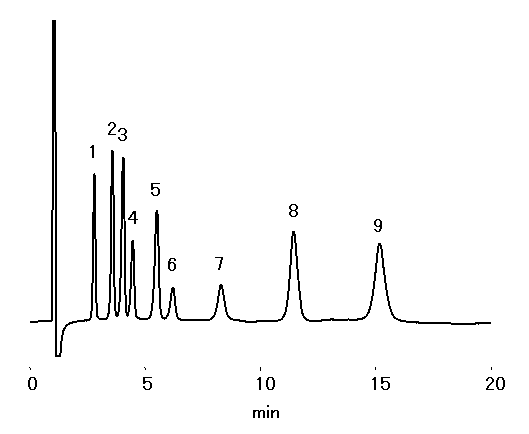
Sample : 10micro-L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. Methylamine 10mg/L
5. K+ 20mg/L
6. Trimethylamine, TMA 20mg/L
7. Triethylamine, TEA 20mg/L
8. Mg2+ 10mg/L
9. Ca2+ 20mg/L
|
Correlation Coefficient(R2)
1mg/L to 100mg/L |
|
| Methylamine | 0.9997 |
| Trimethylamine | 0.9999 |
| Triethylamine | 0.9997 |
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Analysis of mineral water was performed using IC YS-50 (a column for cation chromatography). It turns out that many divalent cations is contained in mineral water.
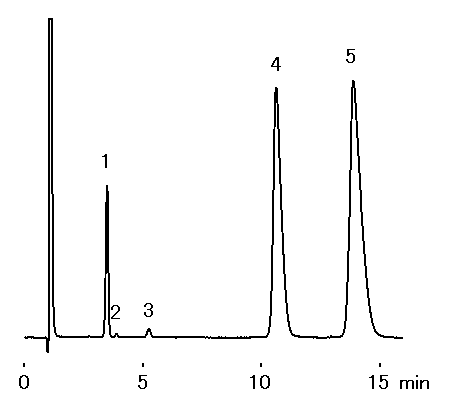
Sample : Mineral water (Water), 10micro-L
1. Na+
2. NH4+
3. K+
4. Ca2+
5. Mg2+
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
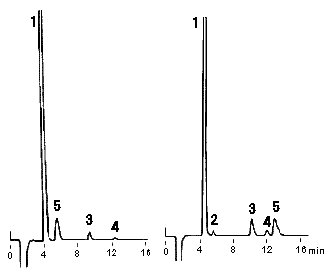
Sea water diluted 100 times was analyzed using IC YS-50 (a column for cation chromatography) by non-supprresor method. YS-50 are suitable for analysis of NH4+ at a high concentration of Na+, such as sea water.
Please refer to Monovalent and Divalent Cation Standards (6) (Comparison of YS-50 and YK-421)
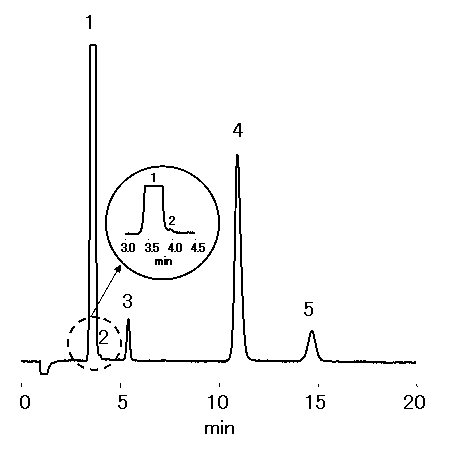
Sample : 100 fold diluted Sea water (Water),
10micro-L
1. Na+
2. NH4+
3. K+
4. Mg2+
5. Ca2+
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Red wine diluted 20 times was analyzed using IC YS-50 (a column for cation chromatography) by non-supprresor method. Monoethanolamine other than Na+, NH4+, K+, Mg2+ and Ca2+ was detected.
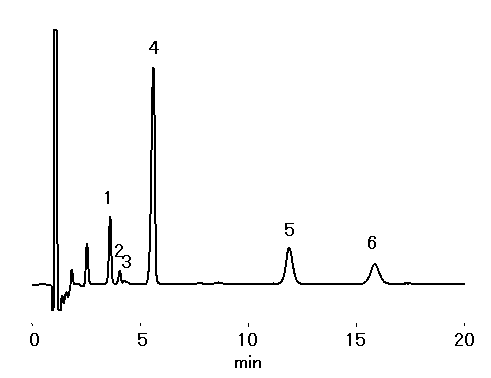
Sample : 20 fold diluted Red wine,
10micro-L
1. Na+
2. NH4+
3. Monoethanolamine
4. K+
5. Mg2+
6. Ca2+
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Waste water from electronic production factories may contain hydrophilic polymers. As a waste water model, sample containing mono- and di-valent cations in the presence of polyethylene glycol (PEG2000) was prepared. Ion chromatography (cation) column, ICYS-50 was used under non-suppressor method. All cations analyzed showed good recovery without having any effect from polyethylene glycol. Some PEG2000 is adsorbed on the column, however this can be removed by adding 50% ACN in the eluent. .
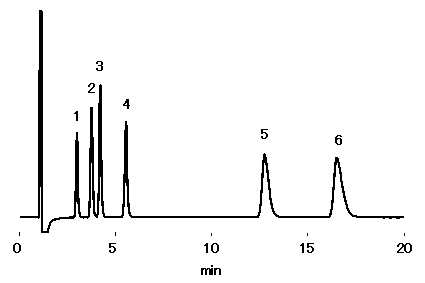
Sample :10micro-L
Cation mixture in 5% PEG2000 solution
|
No.
|
cation
|
Recovery (%)
|
| 1. | Li+ 2mg/L |
106
|
| 2. | Na+ 10mg/L |
104
|
| 3. | NH4+ 10mg/L |
101
|
| 4. | K+ 20mg/L |
101
|
| 5. | Mg2+ 10mg/L |
99
|
| 6. | Ca2+ 10mg/L |
97
|
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Ion chromatography (cation) column, IC YS-50 was used to separate mono- and di-valent cations in the presence of albumin. No considerable influence from the presence of albumin was observed. Part of protein (alubumin) may be adsorbed on the column, thus column cleaning using10mM Na2HPO4(pH9-9.5) is recommended after the analysis.
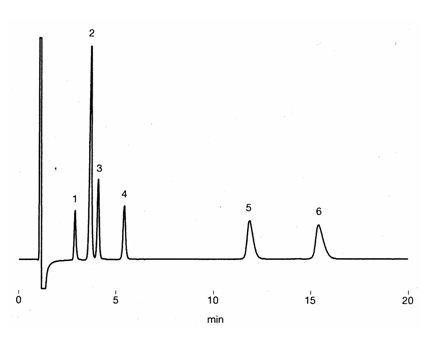
Sample : 10micro-L
Cation mixture in 7% Albumin
1. Li+
2. Na+
3. NH4+
4. K+
5. Mg2+
6. Ca2+
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Monovalent and divalent cations were analyzed using IC YS-50. 18-Crown 6- Ether will make a complex with cations, whose complex building depends on the kind of cations. Therefore, adding 18-Crown-6-Ether is able to control the elusion of cations and improve the separation of Na+ and NH4+.
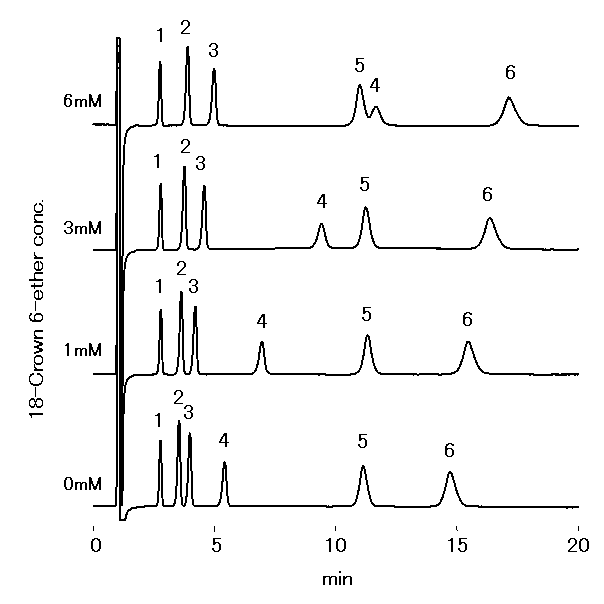
Sample : 10micro-L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. K+ 20mg/L
5. Mg2+ 10mg/L
6. Ca2+ 20mg/L
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Methanesulfonic acid + nmM 18-Crown 6-ether aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
Chloric acid should be analyzed by ion chromatography for water quality standard in accordance to the Ministry of Health, Labor and Welfare in Japan. 1mL of Ethylendiamine (EDA) solution (50mg/mL) is to be added into 1L sample, to prevent generation of chloric acid through the reaction between chlorous acid and residual chlorine. Monovalent and divalent cations in river water were analyzed by IC YS-50.
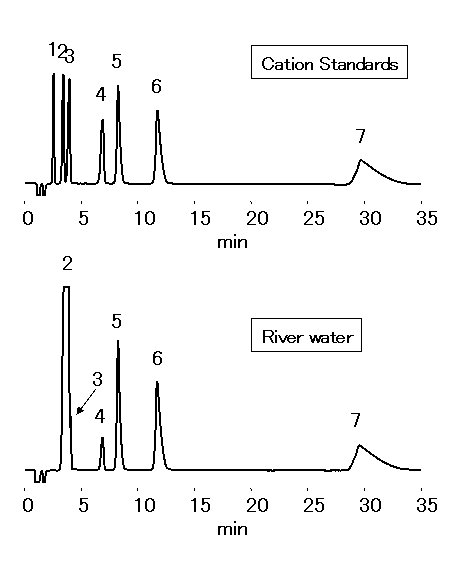
Sample : 50micro-L
Cation std. containing EDA 50mg/L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. K+ 20mg/L
5. Mg2+ 10mg/L
6. Ca2+ 20mg/L
7. Ethylenediamine, EDA 50mg/L
Sample : 50micro-L
River water (Water) containing EDA 50mg/L
2. Na+
3. NH4+
4. K+
5. Mg2+
6. Ca2+
7. Ethylenediamine, EDA 50mg/L
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Nitric acid + 1.5mM 18-Crown 6-ether aq./CH3CN=90/10 Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
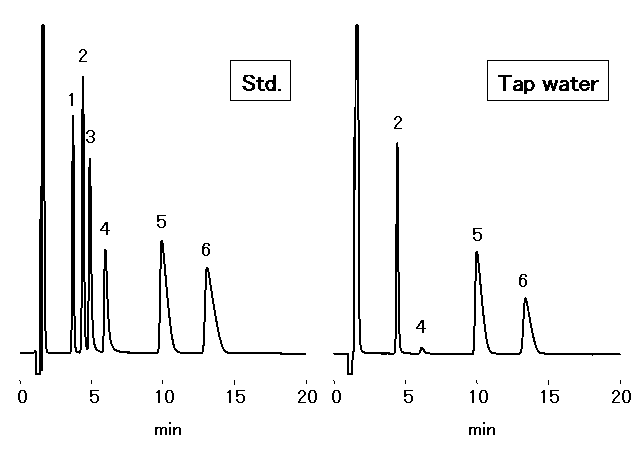
Chloric acid should be analyzed by ion chromatography for water quality standard in accordance to the Ministry of Health, Labor and Welfare in Japan. 1mL of Ethylendiamine (EDA) solution (50mg/mL) is to be added into 1L sample, to prevent generation of chloric acid through the reaction between chlorous acid and residual chlorine. Monovalent and divalent cations in tap water were analyzed by IC YS-50.
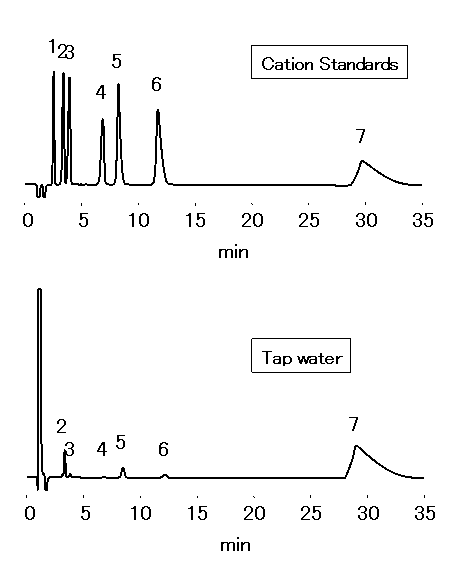
Sample : 50micro-L
Cation std. containing EDA 50mg/L
1. Li+ 2mg/L
2. Na+ 10mg/L
3. NH4+ 10mg/L
4. K+ 20mg/L
5. Mg2+ 10mg/L
6. Ca2+ 20mg/L
7. Ethylenediamine, EDA 50mg/L
Sample : 50micro-L
Tap water (Water) containing EDA 50mg/L
2. Na+
3. NH4+
4. K+
5. Mg2+
6. Ca2+
7. Ethylenediamine, EDA 50mg/L
Column : Shodex IC YS-50 (4.6mmID*125mm) Eluent : 4mM Nitric acid + 1.5mM 18-Crown 6-ether aq./CH3CN=90/10 Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 40deg-C
(IC YK-521 Column)
1. 5mM tartaric acid + 1mM dipicolinic acid Eluent + 1.5g/L boric acid
The eluent of 5mM tartaric acid + 1mM dipicolinic acid + 1.5g/L boric acid is the one generally used for the analysis of cations such as Na+, NH4+, K+, Ca2+ and Mg2+ which are most commonly contained in actual samples. Although Mn2+ elute just after K+, all the other transition metal ions elute at Vo.
Separation can be adjusted by changing the eluent concentration as follows :
* Tartaric acid——5mM is used most commonly. The use of higher concentrations such as 6mM to 7mM, causes cations to elute faster.
* Dipicolinic acid—1mM is used most commonly. It is added to make divalent cations elute faster.
At a higher temperature such as 50 deg-C, Sr2+, Ba2+ elute faster.
(Preparation of eluent)
1) Put 0.750g of reagent grade L(+)tartaric acid, 0.167g of reagent grade dipicolinic acid and 1.5g of reagent grade boric acid into a messflask.
2) Dilute with purified water to make up a 1 liter solution.
3) Dissolve applying ultrasonic vibration.
4) Pass through a 0.2micro-m membrane filter before use.
(Remark) Since tartaric acid deteriorates so fast, this eluent should be prepared just before the use.
2. Addition of crown ether
The separation between Na+ and NH4+ can be largely improved by adding crown ether in the eluent of 5mM tartaric acid + 1mM dipicolinic acid + 1.5g/L boric acid.
For example, Na+ and NH4+ in serum of which concentration ratio is 1 : 300 can be separated well.
3. Addition of acetoritorile
Acetonitorile can be added up to 70% in nitric acid aqueous solution. Therefore, in adition to organic cations, ethanolamine and short chain alkylamines which could be analysed using the old version column(YF-421), long chain alkylamines can also be analysed using IC YK-421.
(Dipicolinic acid Eluent)
Monovalent and divalent cation standards were analyzed using IC YK-421.
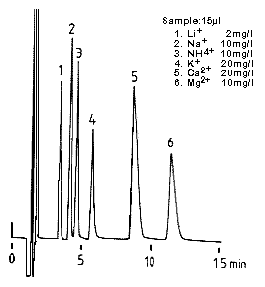
Sample :
1. Li+
2. Na+
3. NH4+
4. K+
5. Ca2+
6. Mg2+
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
Monovalent and divalent cation standards were analyzed using IC YK-421.
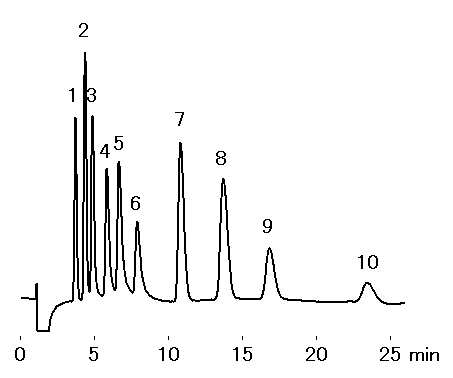
Sample : 10micro-L
1. Li+ 1mg/L
2. Na+ 5mg/L
3. NH4+ 5mg/L
4. K+ 10mg/L
5. Rb+ 30mg/L
6. Cs+ 30mg/L
7. Ca2+ 10mg/L
8. Mg2+ 5mg/L
9. Sr2+ 10mg/L
10. Ba2+ 10mg/L
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 50deg-C
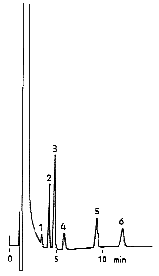
Cations in tap water were analyzed using IC YK-421. Ion chromatogaraphy is used for in Japanese regulations for tap water. Ion chromatography is very useful method for the simultaneous analysis of monovalent and divalent cations such as Na+, K+ and Mg2+.
Sample : 15micro-L
|
No.
|
std.
|
|
|
1
|
Li+ 2mg/L |
–
|
|
2
|
Na+ 10mg/L | Na+ |
|
3
|
NH4+ 10mg/L |
–
|
|
4
|
K+ 20mg/L | K+ |
|
5
|
Ca2+ 20mg/L | Ca2+ |
|
6
|
Mg2+ 10mg/L | Mg2+ |
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
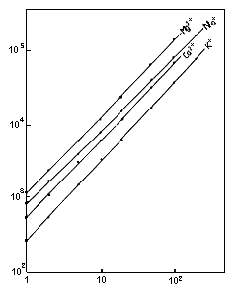
Monovalent and divalent cation standards were analyzed using IC YK-421. This data shows the change in dynamic range of the column with the height of the peaks. There is the linearity in the 1 to 200mg/L range for Na+ and K+ and in the 1 to 100mg/L range for Ca2+ and Mg2+.
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
Monovalent and divalent cation standards were analyzed using IC YK-421. Using a highly sensitive conductivity detector, cations whose concentrations are micro-g/L(=ppb) level can be detected. Minimum detection levels are shown in the table(S/N=3). The figures in parentheses are minimum concentrations when 100 micro-L of sample is injected.
|
Sample
|
Minimum detection levels (S/N=3)
|
||
| 1. Li+ | – | – | |
| 2. Na+ | 0.15ng |
(1.5 micro-g/L)
|
|
| 3. NH4+ | 0.30ng |
( 3 micro-g/L)
|
|
| 4. K+ | 0.60ng |
( 6 micro-g/L)
|
|
| 5. Ca2+ | 0.20ng |
( 2 micro-g/L)
|
|
| 6. Mg2+ | 0.20ng |
( 2 micro-g/L)
|
|
|
( ) : minimum concentrations when
100 micro-L of sample is injected. |
|||
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
20-fold dilution of red wine was analyzed using IC YK-421. Besides Na+, NH4+, K+, Ca2+ and Mg2+, monoethanolamine was detected.
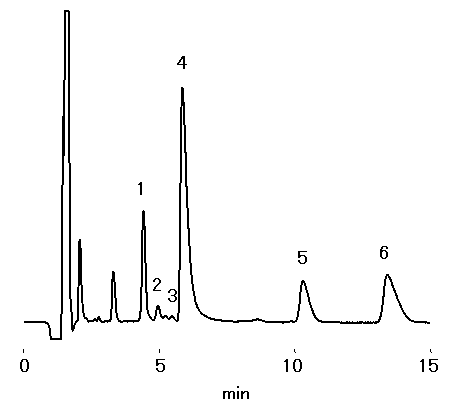
Sample : 20-fold diluted Red Wine, 20micro-L
1. Na+
2. NH4+
3. Monoethanolamine
4. K+
5. Ca2+
6. Mg2+
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
(Dipicolinic acid + Crown ether Eluent)
The problems in the anaysis using IC YK-421 is that the analysis of very small amount of NH4+ in a sample which contains large amount of Na+ is difficult. Addition of 1.5mM 18-Crown 6-ether in 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid eluent can solve the problem. Addition of crown 6-ether can adjust the elution volume of the cations. Especially, the elution volume of K+ is largely affected by the additon and elute after Mg2+.
Sample :
1. Li+
2. Na+
3. NH4+
4. Ca2+
5. Mg2+
6. K+
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid + 1.5mM 18-Crown 6-ether aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
The problems in the anaysis using IC YK-421 is that the analysis of very small amount of NH4+ in a sample which contains large amount of Na+ is difficult. Addition of 1.5mM 18-Crown 6-ether in 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid eluent can solve the problem. Addition of crown 6-ether can adjust the elution volume of the cations. Especially, the elution volume of K+ is largely affected by the additon and elute after Mg2+.
Sample : Serum
1. Na+
2. NH4+
3. Ca2+
4. Mg2+
5. K+
Column : Shodex IC YK-421 (4.6mmID*125mm)
Eluent : (left) 5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid
(right)5mM Tartaric acid + 1mM Dipicolinic acid + 1.5g/L Boric acid + 1.5mM 18-Crown 6-ether
Flow rate : 1.0mL/min
Detector : Non-suppressed coductivity
Column temp. : 40deg-C
(Nitric acid + Acetoritorile Eluent)
Monovalent and divalent cation standards were analyzed using IC YK-421. The effect of the acetonitorile concentration in 3mM nitric acid on elution volume of cations is shown. By increasing the acetonitorile concentration, the cations are eluted faster. Especially, the elution volume of divalent cations are eluted faster markedly. When the acetonitorile concentration of acetonitrile is higher than 50%, to the contrary, cations are eluted slower a little by increasing acetonitorile concentration.
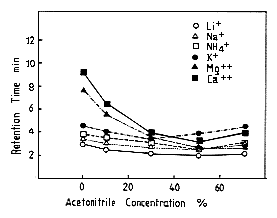
Sample :
Li+
Na+
NH4+
K+
Mg2+
Ca2+
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 3mM Nitric acid aq./CH3CN Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
Long chain alkylamines such as TBA (Tributylamine) and TPA (Tripropylamine) were analysed by ion-pair method using RI detector. However, this method is not so sensitive. Using IC YK-421 with nitric acid aqueous solution/ acetonitrile eluent and a conductivity detector, highly sensitive analysis of long chain alkylamines can be realized.The effect of acetonitrile concentration on elution volume of alkylamines are shown. When the acetonitrile concentration is lower than 50%, the components which have shorter chain elute faster. And, to the contrary, when the acetonitrile concentration if higher than 50%, the components which have longer chain elute faster. The reason of the difference can be explained by the the difference of the separation mode. When acetonitrile concentration is lower than 50%, the separation mode is hydrohorbic interaction, and, when acetonitrile concentration is higher than 50% the separation mode is ion exchange.
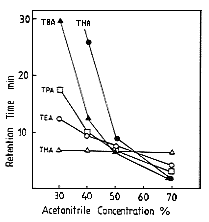
Sample : Alkylamines
THA, Trihexylamine
TBA, Tributylamine
TPA, Tripropylamine
TEA, Triethylamine
TMA, Trimethylamine
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 3mM Nitric acid aq./CH3CN Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
Trialkylamines were separated using cation chromatography column, IC YK-421. By using the mobile phase stated below, hydrophobic interaction dominates ioninc-interaction. Therefore, shorter chain alkyl amines (less hydrophobic) will elute before longer ones.
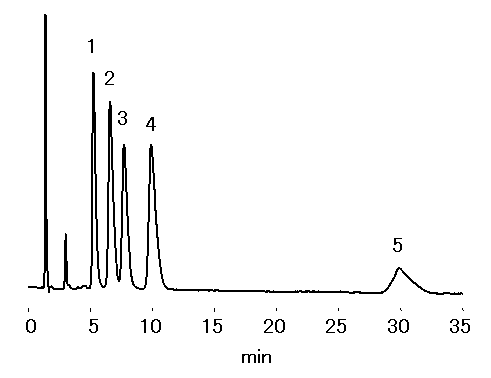
Sample : Alkylamines 100mg/L, 20micro-L
1. Trimethylamine, TMA
2. Triethylamine, TEA
3. Tripropylamine, TPA
4. Tributylamine, TBA
5. Trihexylamine, THA
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 4mM HNO3 aq./CH3CN=70/30 Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
Trialkylammonium were separated using cation chromatography column, IC YK-421. By using the mobile phase used below (high ACN concentration), hydrophobic interaction is suppressed. Thus ioninc-interaction dominates, this makes shorter chain alkylammonium to be retained longer than longer ones.
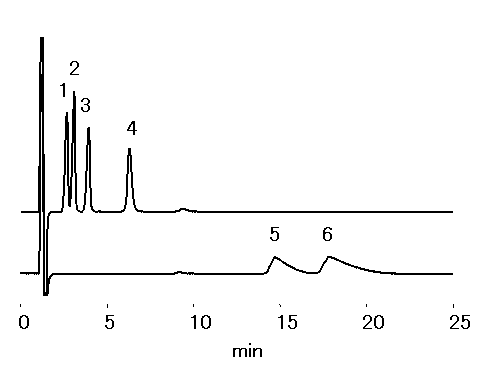
Sample : 100mg/L each, 20micro-L
1. Tetrahexylammonium
2. Tetrapentylammonium
3. Tetrabutylammonium
4. Tetrapropylammonium
5. Tetraethylammonium
6. Tetramethylammonium
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 2.5mM HNO3 aq./CH3CN=30/70 Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 40deg-C
(Phosphoric acid Eluent)
Monovalent and divalent cation standards were analyzed using IC YK-421.
Please refer to YK-421 (tartaric acid + dipicolinic acid eluent)
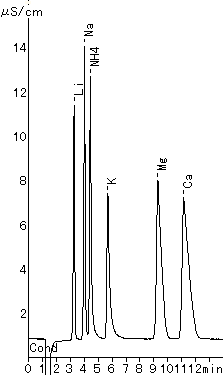
Sample : 20micro-L
1. 2mg/L Li+
2. 10mg/L Na+
3. 10mg/L NH4+
4. 20mg/L K+
5. 10mg/L Mg2+
6. 20mg/L Ca2+
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 4mM H3PO4 aq. Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 25deg-C
Monovalent and divalent cations in tap water were analyzed using IC YK-421.
Please refer to YK-421 (tartaric acid + dipicolinic acid eluent) and YK-421 (tartaric acid + dipicolinic acid eluent)
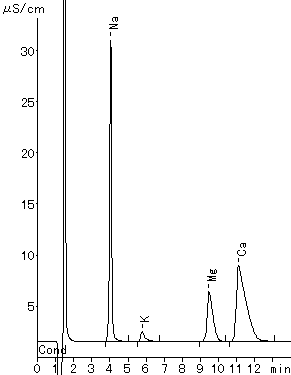
Sample : 20micro-L Tap water (Water)
1. Na+
2. K+
3. Mg2+
4. Ca2+
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 4mM H3PO4 aq. Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 25deg-C
With using IC YK-421, when Na+ is contained so much in a sample, analysis of very small quantity of NH4+ is difficult. Separation of Na+ and NH4+ is improved by adding 18-Crown 6-etherb in phosphate acid aqueous as eluent.
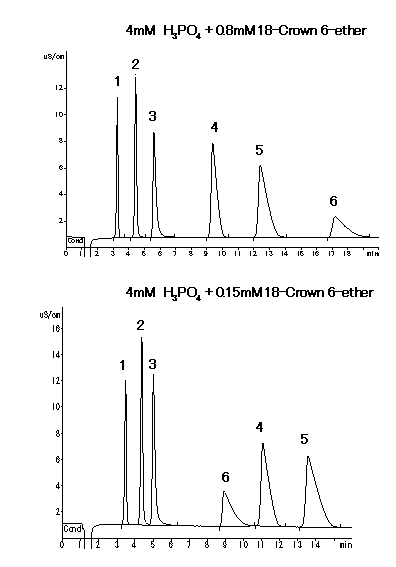
Sample : 20micro-L
1. 2mg/L Li+
2. 10mg/L Na+
3. 10mg/L NH4+
4. 20mg/L Ca2+
5. 10mg/L Mg2+
6. 20mg/L K+
Column : Shodex IC YK-421 (4.6mmID*125mm)
Eluent : (above); 4mM H3PO4 + 0.8mM 18-Crown 6-ether aq.
(below); 4mM H3PO4 + 0.15mM 18-Crown 6-ether aq.
Flow rate : 1.0mL/min
Detector : Non-suppressed conductivity
Column temp. : 25deg-C
Alkylamines were analyzed using IC YK-421. It is possible to analyze alkylamines with short time and high sensitively, when acetonitrile was added to the eluent. Because hydrophobic cations (ex. Alkylamines) are eluted earlier with the addition of acetnitile to the eluent. Phosphate eluent is suitable for the analysis.
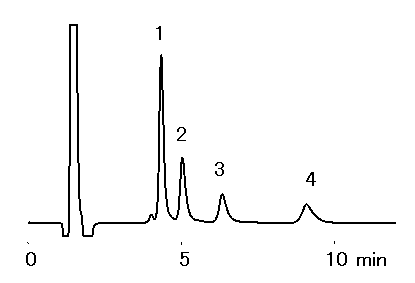
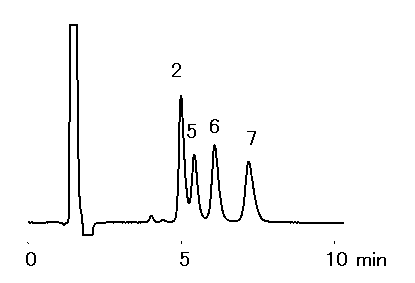
Sample : 50micro-L
1. NH4+ 5mg/L
2. Methylamine, MA 5mg/L
3. Dimethylamine, DMA 5mg/L
4. Trimethylamine, TMA 20mg/L
Sample : 10mg/L each, 50micro-L
2. Methylamine, MA
5. Ethylamine, EA
6. Propylamine, PA
7. Butylamine, BA
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 4mM H3PO4 aq./CH3CN=90/10 Flow rate : 1.0mL/min Detector : Non-suppressed conductivity Column temp. : 25deg-C
Ethanolamines and alkylamines were analyzed using IC YK-421. Sharp peaks were obtained during the analysis of ethanolamines, but during the analysis of alkylamines, peaks with a little tailing were observed.
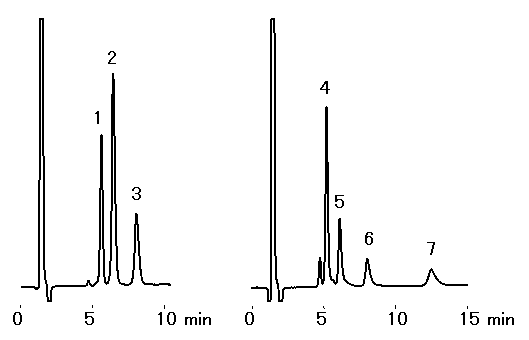
Sample : 50micro-L
1. Monoethanolamine 10mg/L
2. Diethanolamine 10mg/L
3. Triethanolamine 10mg/L
4. NH4+ 5mg/L
5. Methylamine 5mg/L
7. Dimethylamine 5mg/L
8. Trimethylamine 20mg/L
Column : Shodex IC YK-421 (4.6mmID*125mm) Eluent : 4mM H3PO4 aq./CH3CN=95/5 Flow rate : 1.0mL/min Detector : Non-suppressed coductivity Column temp. : 25deg-C
