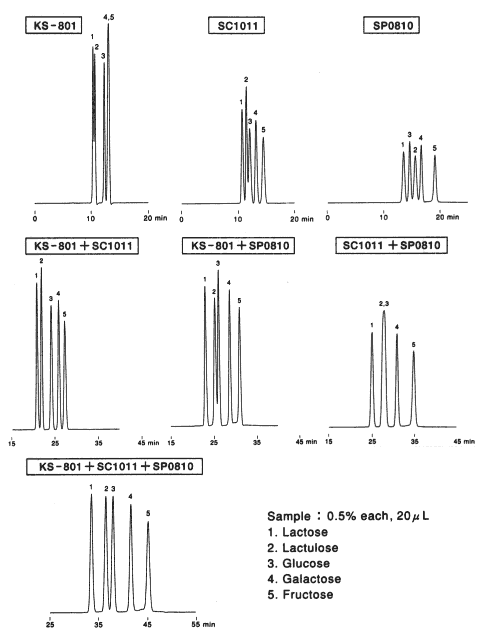
Monosaccharides and Oligosaccharides
Elution volumes of saccharides for seven different columns, SUGAR SP0810, SC1011, KS-801, SZ5532, SC1211, RSpak DC-613, Asahipak NH2P-50 4E and GS-220 HQ are shown in the table. As only the peak top position can be obtained from the table, if you want to to see the chromatogram with actual peak width, please contact us.
| Substances | Elution Volume (mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SUGAR | RSpak | Asahipak | |||||||
| SP0810 | SC1011 | KS-801 | SZ5532 | SC1211 | DC-613 | NH2P-50 4E | GS-220 HQ x 2 | ||
| Pb2+ | Ca2+ | Na+ | Zn2+ | Ca2+ | Na+ | ||||
| N-Acetyl-alpha -D-glucosamine |
8.86 | 7.75 | 6.68 | 2peaks | 4.10 | 2peaks | 6.66 | ||
| D(+)-Arabinose | 10.42 | 8.91 | 8.21 | 5.11 | 5.56 | 5.75 | 6.18 | ||
| D-Arabitol | 15.86 | 11.33 | 7.63 | 7.27 | 8.16 | 5.81 | 6.29 | ||
| Aspartame | – | – | – | – | 34.02 | – | – | ||
| 2-Deoxy-D-glucose | 8.83 | 7.58 | 7.15 | 4.34 | 4.02 | 4.43 | 6.02 | ||
| Difructose anhydride III | 7.07 | 6.30 | 5.81 | 4.30 | * | 4.51 | 7.77 | ||
| Dulcitol | 20.18 | 12.76 | 7.40 | 9.46 | 11.28 | 7.33 | 7.45 | ||
| meso-Erythritol | 12.70 | 10.09 | 7.86 | 5.73 | 6.27 | 4.84 | 5.43 | 17.38 | |
| Ethanol | 11.13 | 11.33 | 10.09 | – | 4.27 | – | – | ||
| 1-Fructofuranosyl -D-nystose |
6.05 | 5.27 | 4.76 | 31.43 | * | – | – | 14.24 | |
| D(-)-Fructose | 11.05 | 8.85 | 7.71 | 5.37 | 5.90 | 6.19 | 6.75 | 17.05 | |
| D(+)-Fucose | 10.48 | 8.84 | 8.09 | 4.50 | 4.96 | 4.81 | 5.43 | ||
| D(+)-Galactose | 9.74 | 7.98 | 7.58 | 6.46 | 4.98 | 7.28 | 8.10 | 16.60 | |
| 4′-Galactosyllactose | 7.42 | 6.02 | 5.40 | 19.02 | * | 21.24 | 21.66 | ||
| alpha-D-Galacturonic acid | – | 6.28 | 4.36 | – | 5.63 | – | – | ||
| Gentiobiose | 7.22 | 6.08 | 5.75 | 10.50 | * | 13.18 | 16.36 | ||
| Glucose | 8.63 | 7.30 | 7.17 | 5.87 | 4.76 | 6.83 | 8.61 | 16.75 | |
| Glycerol | 4.26 | 17.83 | |||||||
| Glycyrrhizin | – | – | – | – | 2.71 | 2.00 | – | ||
| myo-Inositol | 12.77 | 8.86 | 7.99 | 12.63 | 7.87 | 15.80 | 9.96 | ||
| Isomaltose | 7.68 | 6.26 | 5.95 | 10.57 | * | 13.82 | 15.18 | 15.57 | |
| Isomaltotriose | 7.09 | 5.75 | 5.34 | 21.17 | * | 32.02 | 27.55 | 14.72 | |
| 1-Kestose | 6.79 | 5.75 | 5.26 | 13.09 | * | – | 20.11 | 15.31 | |
| Kojibiose | 7.56 | 6.21 | 5.88 | 9.65 | * | 11.47 | 14.82 | ||
| Lactitol | 13.27 | 8.09 | 6.13 | 16.35 | 6.67 | 14.04 | 11.82 | 15.50 | |
| Lactose | 8.05 | 6.51 | 5.99 | 10.12 | 4.07 | 11.69 | 13.27 | 15.71 | |
| Lactosylfructoside | 7.12 | 5.88 | 5.29 | 14.69 | * | 15.75 | 18.98 | ||
| Lactulose | 9.13 | 6.99 | 6.19 | 9.16 | 4.65 | 10.80 | 10.72 | ||
| Maltitol | 12.23 | 8.26 | 6.03 | 13.04 | 6.77 | 11.81 | 11.82 | 15.82 | |
| Maltoheptaose | – | 14.82 | |||||||
| Maltohexaose | – | 14.56 | |||||||
| Maltopentaose | – | 14.31 | |||||||
| Maltose | 7.85 | 6.34 | 5.94 | 8.67 | * | 10.61 | 14.24 | 16.11 | |
| Maltotriose | 7.48 | 5.89 | 5.38 | 13.79 | * | 17.88 | 24.96 | 15.56 | |
| Mannitol | 15.80 | 11.10 | 7.23 | 8.75 | 9.03 | 6.84 | 7.39 | ||
| D-Mannose | 10.72 | 8.17 | 7.64 | 5.83 | 5.01 | 6.72 | 7.84 | ||
| D(+)-Melezitose | 6.94 | 5.79 | 5.24 | 13.60 | * | 14.68 | 19.27 | ||
| Melibiose | 8.16 | 6.45 | 5.98 | 11.69 | 4.23 | 14.83 | 14.70 | ||
| Methyl-alpha -D-mannopyranoside |
11.13 | 8.87 | 7.78 | 3.99 | 4.39 | 4.15 | 4.71 | ||
| Nystose | 6.38 | 5.45 | 4.93 | 20.05 | * | – | 31.90 | 14.72 | |
| Palatinit | 2peaks | 2peaks | 5.90 | 2peaks | 2peaks | 2peaks | 12.73 | ||
| Palatinose | 7.84 | 6.45 | 5.89 | 8.08 | 3.99 | 9.81 | 12.12 | ||
| Panose | 7.14 | 5.78 | 5.32 | 16.87 | * | 23.14 | 25.60 | ||
| L-Phenylalanine | – | – | – | – | 31.58 | – | – | ||
| D(+)-Raffinose | 7.14 | 5.78 | 5.29 | 16.36 | * | 19.11 | 20.25 | 15.08 | |
| D(+)-Rhamnose | 9.77 | 8.23 | 7.37 | 3.93 | 4.43 | 4.09 | 5.52 | ||
| D(-)-Ribose | 19.35 | 13.66 | 9.04 | 4.82 | 8.64 | 5.30 | 5.45 | ||
| Rutinose | 7.81 | 6.49 | 5.80 | 6.65 | * | 7.21 | 10.87 | ||
| Saccharin sodium | 6.96 | 5.44 | 4.33 | 6.77 | * | 2.04 | 5.68 | ||
| D(-)-Sorbitol | 21.61 | 13.31 | 7.42 | 9.79 | 11.88 | 7.27 | 7.09 | 16.60 | |
| D(+)-Sorbose | 9.67 | 8.03 | 7.38 | 5.12 | 4.92 | 5.91 | 7.35 | ||
| Stachyose | 6.82 | 5.57 | 4.97 | – | * | – | 36.22 | 14.33 | |
| Stevioside | – | – | – | 4.14 | * | 4.44 | 6.07 | ||
| Sucrose | 7.54 | 6.29 | 5.87 | 7.91 | * | 8.68 | 11.87 | 16.26 | |
| alpha-D-Talose | 21.33 | 12.59 | 8.76 | 5.69 | 8.51 | 6.32 | 6.47 | ||
| Theanderose | 2peaks | 2peaks | 2peaks | 2peaks | * | 2peaks | |||
| Trehalose | 7.62 | 6.27 | 5.78 | 10.85 | * | 11.49 | 13.25 | ||
| Trehalulose | 8.92 | 6.95 | 6.10 | 9.54 | 4.78 | 11.38 | 11.68 | ||
| Xylitol | 19.87 | 13.14 | 7.94 | 7.77 | 10.16 | 6.19 | 6.10 | 16.88 | |
| Xylobiose | 8.16 | 6.68 | 6.40 | 5.65 | * | 6.71 | 9.05 | ||
| D(+)-Xylose | 9.21 | 7.90 | 7.71 | 4.55 | 4.48 | 5.21 | 6.58 | 17.46 | |
| D-Xylulose | 10.64 | 9.02 | 8.04 | 4.06 | 5.07 | 4.56 | 5.41 | ||
(-)–>cannot be detected
(*)–>cannot be separated from solvent peak
Columns : Shodex SUGAR SP0810, SC1011, KS-801 (8.0mmID*300mm each) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C Column : Shodex SUGAR SZ5532 (6.0mmID*150mm) Eluent : H2O/CH3CN=25/75 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 60deg-C Column : Shodex SUGAR SC1211 (6.0mmID*250mm) Eluent : H2O/CH3CN=65/35 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 70deg-C Column : Shodex RSpak DC-613 (6.0mmID*150mm) Eluent : H2O/CH3CN=25/75 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 70deg-C Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : H2O/CH3CN=25/75 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C Column : Shodex Asahipak GS-220 HQ (7.5mmID*300mm) x 2 Eluent : H2O Flow rate : 0.5mL/min Detector : Shodex RI Column temp. : 60deg-C
After reduction of the sugar and saccharide, the terminal groups may have various types of cyclic forms in addition to the open-chain form. In such cases, these saccharides exist as two diastereomers because the carbon atom of the terminal reducing group is asymmetric; these diastereomers are called anomers (alpha- and beta-anomers respectively). Under certain conditions where the rate of conversion of such diastereomers is low, alpha-anomer and beta-anomer are separated as they pass through the column. This causes a undesirable splitting or broadening of the peak. For the analysis of sugars, therefore, it is necessary to prevent the anomer separation. Below are possible methods for controlling this separation:
1) It has been reported that the anomer separation does not take place at high temperature. Therefore, when using SUGAR series (SP0810, SC1011, SC1211 or KS-800), set up the column temperature at 70 to 80deg-C.
2) It has been reported that the anomer separation does not take place under strong alkaline conditions. Polymer-base columns such as NH2P allow the separation of saccharides without causing the anomer separation as the column can be used under alkaline environment.
3) For other columns, refer to the conditions described in the chromatograms.
It has been reported that the anomer separation does not take place at high temperature. Therefore, when using SUGAR series (SP0810, SC1011, KS-801, SZ5532 or SC1211), set up the column temperature at 70-80 deg-C.
Please refer to Prevention of Anomer Separation.
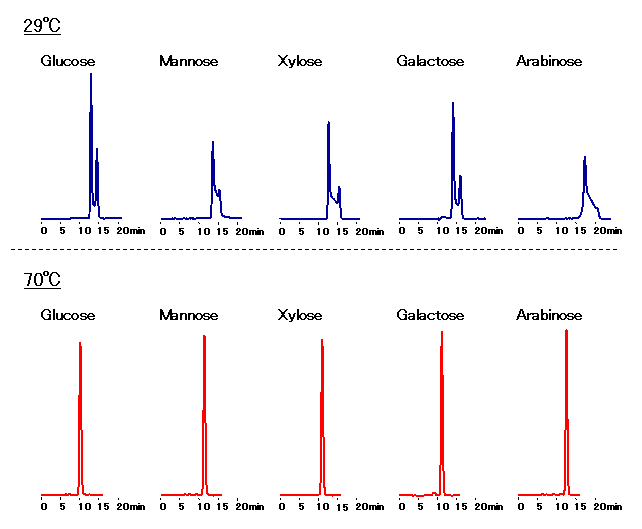
Sample : 0.5% each, 10micro-L
Glucose, Mannose, Xylose, Galactose, Arabinose
Column : Shodex SUGAR SC1011 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.7mL/min Detector : Shodex RI Column temp. : 29deg-C, 70deg-C
It has been reported that the anomer separation does not take place under strong alkaline conditions. Amino columns such as Asahipak NH2P-50 4E allow the separation of saccharides without causing the anomer separation as they can be used under alkaline conditions at room temperature.(Please refer to Prevention of Anomer Separation.)
Amide columns are also used for the separation of saccharides. However, acryl-amide goups are not alkaline, it is necessary to perform the separation at elevated temperature to prevent anomer separation.
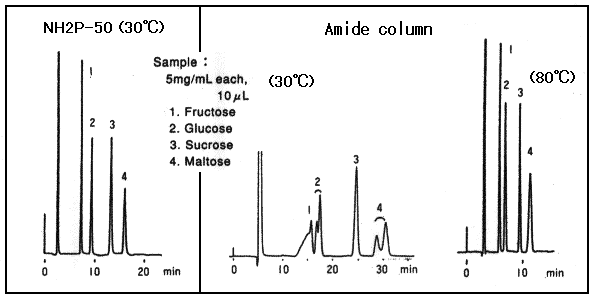
Sample : 5mg/mL each, 10micro-L
1. Fructose
2. Glucose
3. Sucrose
4. Maltose
Column : Shodex Asahipak NH2P-50 4E, Amide column from other manufacturer (4.6mmID*250mm each) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
Comparison of columns with different counter ions, Pb2+ (SP0810), Ca2+ (SC1011) and Na+ (KS-801) is shown. For the separation of monosaccharides(glucose and fructose), the separation by KS-801 is not sufficient because the separation mode of KS-801 is mainly GFC mode and their molecular weights are too close to be separated by GFC mode. When SP0810 and SC1011 are used, monosaccharides can be separated well because ligand exchange mode works for these columns.
For the separation of oligosaccharides(maltotriose and maltose), KS-801 is the best suited. The difference is caused by the difference of peak width. In case of SC1011 and SP0810, ligand exchange mode works and anomers are separated by the mode. And, as the consequence, the peak width become larger. Since the ligand exchange mode of SP0810 is stronger than that of SC1011, the separation of oligosaccharides by SP0810 is the worst.
As the conclusion, we can say that monosaccharides can be separated well by ligand exchange mode and oligosaccharides can be separated well by GFC mode.
Sugar alcohol(sorbitol) is separated by the ligand exchange mode. In case of SP0810, the elution of sorbitol is too slow because ligand exchange mode is working too strongly.
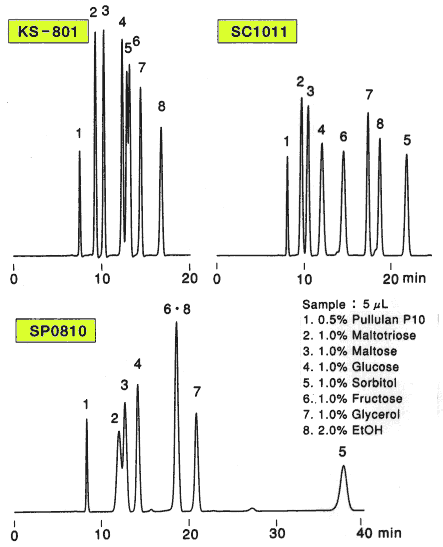
Sample :
1. Pullulan
2. Maltotriose
3. Maltose
4. Glucose
5. Sorbitol
6. Fructose
7. Glycerol
8. Ethanol
Columns : Shodex SUGAR SP0810, SC1011, KS-801 (8.0mmID*300mm each) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 80deg-C
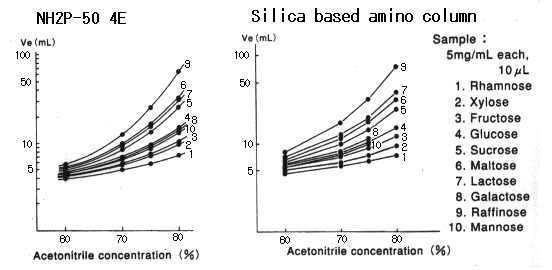
When Asahipak NH2P-50 columns are used, the retention time of sugar alcohols as well as saccharides becomes longer as the acetonitrile concentration in the eluent becomes higher.
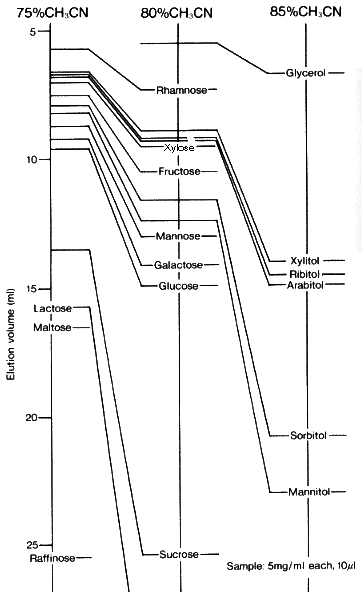
Sample :
Arabitol
Fructose
Galactose
Glucose
Glycerol
Lactose
Maltose
Mannitol
Mannose
Raffinose
Rhamnose
Ribitol
Sorbitol
Sucrose
Xylitol
Xylose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
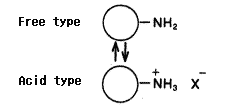
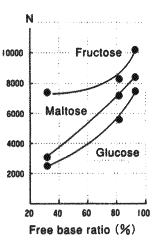
Same as packing materials for silica based amino columns, the packing material for Asahipak NH2P-50 columns has amino groups which are bonded to weak anion exchange resins.
The amino groups exist in the equilibrium of free type and acid type. (Please refer to the right figure.) The state of the equilibrium(free base ratio) depends on pH and ionic composition of the eluent.
To investigate the effect of free base ratio, NH2P-50 4E columns are equilibrated with ammonium acetate solvents of different pH to prepare columns of different free base ratio. Using the columns, analyses were performed under the same conditions. The result shows that the elution times became shorter and peak shapes became sharper as free base ratio increased.
.
| pH | 2.84 | 8.50 | 9.30 | Plate number vs free base ratio |
|---|---|---|---|---|
| Free base ratio |
32% | 82% | 93% | |
| Chromato- gram |
 |
 |
 |
 |
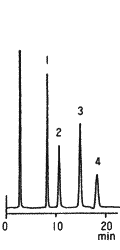
Sample : 1. Fructose, 2. Glucose, 3. Sucrose, 4. Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
The separation of saccharides using Asahipak NH2P-50 column can be controlled by changing mixing ratio of acetonitrile and water in the eluent. Since the NH2P-50 column is free from non-specific adsorption, sharp and symmetrical peaks can be obtained for most of saccharides.
When the NH2P-50 column is used, as same as when silica-based amino columns are used, the retention time of saccharides becomes longer as the acetonitrile concentration in the eluent becomes higher. The elution order of saccharides using the NH2P-50 columns is almost as same as that using silica-based amino columns though there are some exception such as the order between galactose and glucose and between lactose and maltose.
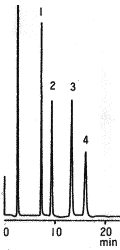
Sample :
1. Rhamnose
2. Xylose
3. Fructose
4. Glucose
5. Sucrose
6. Maltose
7. Lactose
8. Galactose
9. Raffinose
10. Mannose
Columns : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) , Silica based amino column(other manufacturer) Eluent : CH3CN/H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
The base material of the packing material of SUGAR SZ5532 is the gel developed for adsorption & partition mode. It is highly cross-linked and its swelling and shrinkage is very small. The counter ion is Zn2+. Zn2+ forms a complex with saccharides but, when the counter ion is Zn2+, saccharides are not so strongly reteined by ligand exchange mode. And, when organic solvents are added in the eluent, saccharides and sugar alcohols are reteined by adsorption & partition mode and their elution volumes become larger by inceasing organic solvent concentration.
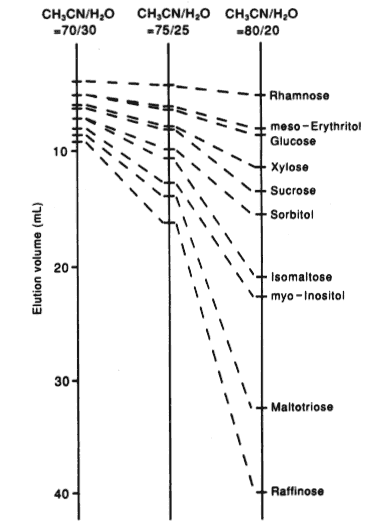
Sample :
Rhamnose
meso-Erythritol
Glucose
Xylose
Sucrose
Sorbitol
Isomaltose
myo-Inositol
Maltotriose
Raffinose
Column : Shodex SUGAR SZ5532 (6.0mmID*150mm) Eluent : CH3CN/H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 60deg-C
When Asahipak NH2P-50 column is used, plate number of saccharides becomes higher as the acetonitrile concentration in the eluent becomes higher.
When silica-based amino columns are used, plate number and detection sensitivity of saccharides are different according to the type of saccharides, and, the plate number of some specific saccharide such as galactose is extremely low.
Please refer to Comparison of NH2P and Silica-based Amino Column (2).
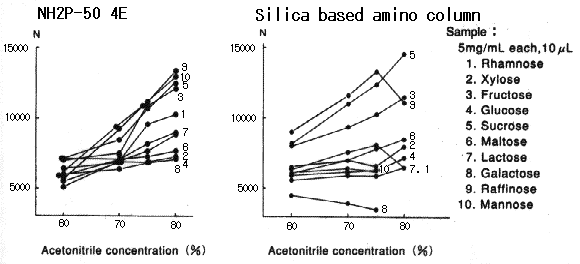
Sample :
1. Rhamnose
2. Xylose
3. Fructose
4. Glucose
5. Sucrose
6. Maltose
7. Lactose
8. Galactose
9. Raffinose
10. Mannose
Columns : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm), Silica based amino column (other manufacturer) Eluent : CH3CN/H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
Testing procedure
(1)Testing(63hrs) Eluent : 0.01M H2SO4(pH2) Flow rate : 0.1mL/min Column temp. : Room temp. |
(2)Replacement(1hr) Eluent : H2O Flow rate : 0.5mL/min Column temp. : Room temp. |
(3)Equilibrium(1hr) Eluent : 0.1M NaOH Flow rate : 0.5mL/min Column temp. : Room temp. |
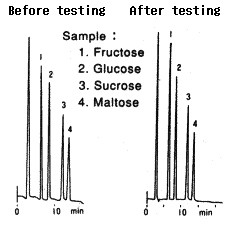
Comparison between before and after testing
| Sample | Before testing | After testing | ||
|---|---|---|---|---|
| Elution volume (mL) |
Plate number | Elution volume (mL) |
Plate number | |
| 1.Fructose | 6.73 | 9600 | 6.70 | 8400 |
| 2.Glucose | 8.59 | 7900 | 8.58 | 8500 |
| 3.Sucrose | 11.88 | 9600 | 11.93 | 9300 |
| 4.Maltose | 14.24 | 8500 | 13.90 | 8600 |
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
Testing procedure
(1)Equilibrium(1hr) Conditions before testing Eluent : 100mM Ammonium acetate(pH9.3) Flow rate : 0.1mL/min Column temp. : Room temp. |
(2)Analysis |
(3)Testing(160hrs) Eluent : 5mM NaOH(pH11.4)/CH3CN=25/75 Flow rate : 0.1mL/min Column temp. : 30deg-C |
(4)Replacement(0.5hr) Eluent : H2O Flow rate : 0.5mL/min Column temp. : Room temp. |
(5)Replacement(0.5hr) Eluent : H2O Flow rate : 0.5mL/min Column temp. : Room temp. |
(6)Analysis |
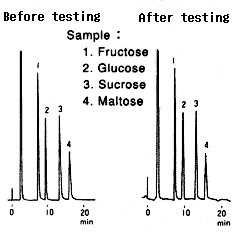
Comparison between before and after testing
| Sample | Before testing | After testing | ||
|---|---|---|---|---|
| Elution volume (mL) |
Plate number | Elution volume (mL) |
Plate number | |
| 1.Fructose | 7.52 | 7100 | 7.62 | 8100 |
| 2.Glucose | 9.63 | 6100 | 9.68 | 7700 |
| 3.Sucrose | 13.41 | 8400 | 13.40 | 9700 |
| 4.Maltose | 16.16 | 7200 | 16.08 | 7400 |
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
Since the swelling and shrinking of the packing material of Asahipak NH2P-50 columns is considerably small even when the eluent composition is changed, any mixing ratio of acetonitrile and water can be used with the NH2P-50 columns.
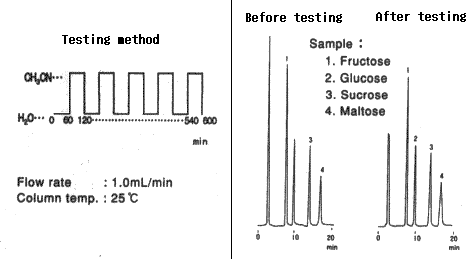
Comparison between before and after testing
| Sample | Before testing | After testing | ||
|---|---|---|---|---|
| Elution volume (mL) |
Plate number | Elution volume (mL) |
Plate number | |
| 1. Fructose | 7.61 | 11600 | 7.56 | 9800 |
| 2. Glucose | 9.80 | 7500 | 9.72 | 6200 |
| 3. Sucrose | 13.86 | 11700 | 13.75 | 9300 |
| 4. Maltose | 16.78 | 8300 | 16.66 | 6700 |
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
When a sample containing polysaccharides is injected into amino columns, polysaccharides are adsorbed by the packing material and it causes poor separation and/or broad peaks.
The figures below show the result when polysaccharide (pullulan, MW: 1,200,000) is injected into the Asahipak NH2P-50 4E column:
1) Figure (A) shows that there is little difference in elution time.
2) Figure (B) shows that the plate number decreses as the amount of adsorption increases.
3) Figure (C) shows the tailing in chromatogram after the adsorption.
Since it is not so easy to eliminate polysaccharides which are adorbed by the packing material, it is neccesary to pre-treat the sample and eliminate polysaccharides before injection.
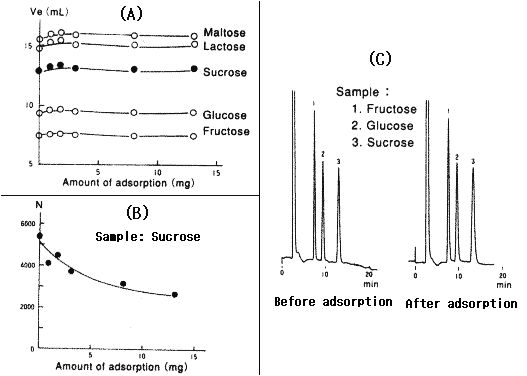
Sample : Maltose, Lactose, Sucrose, Glucose, Fructose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/10mM *Tetrapropylammonium buffer(pH10.0)=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
*How to make Tetrapropylammonium buffer
Please add acetic acid to 10mM Tetrapropylammonium hydroxide and adjust pH10.
Effect of sample solvent composition when Asahipak NH2P-50 is used is shown. When making sample solution to be injected, sample should be dissolved in a solvent whose composition is as close as the eluent, preferably in the eluent. When 75% acetonitrile solvent is used for the eluent and a sample cannot be dissolved in it, first, the sample should be dissolved in a solvent which dissolves the sample, and then, adjust the solvent composition to be acetonitrile aqueous solution of 50% or more.
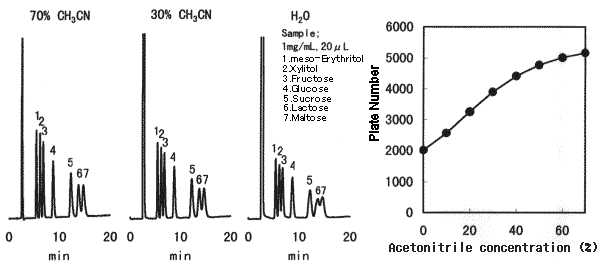
Sample : 1mg/mL each, 20micro-L
1. meso-Erythritol 2. Xylitol 3. Fructose 4. Glucose 5. Sucrose 6. Lactose 7. Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
Effect of sample injection volume when Asahipak NH2P-50 4E is used is shown. When making sample solution to be injected, sample should be dissolved in a solvent whose composition is as close as the eluent, preferably in the eluent. When 75% acetonitrile aqueous solution is used for the eluent and a sample can be dissolved only in aqueous solution (or 50v/v% ethanol, etc.), injection volume should be as small as possible.
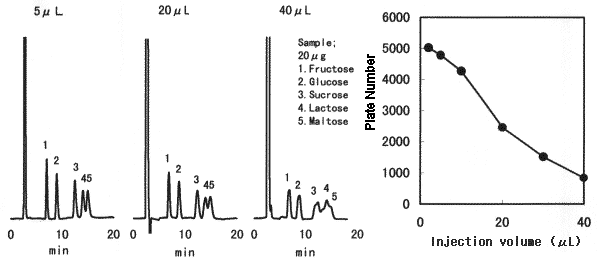
Sample : 20micro-g each in purified water
1. Fructose 2. Glucose 3. Sucrose 4. Lactose 5. Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
Effect of flow rate when analyzing saccharides using SUGAR SC1011 (a column for saccharide analysis) is shown here.
It is confirmed that the theoretical plate number and resolution are improved by decreasing the flow rate.
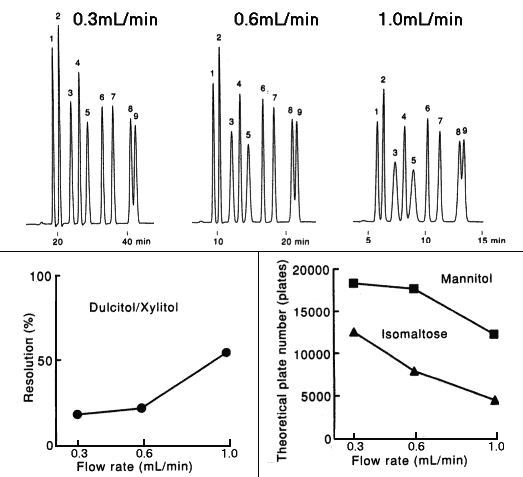
Sample : 0.5%each, 5micro-L
1. Isomaltotriose
2. Sucrose
3. Glucose
4. Lactitol
5. Fructose
6. meso-Erythritol
7. Mannitol
8. Dulcitol
9. Xylitol
Column : Shodex SUGAR SC1011 (8.0mmID*300mm) Eluent : H2O Detector : Shodex RI Column temp. : 80deg-C
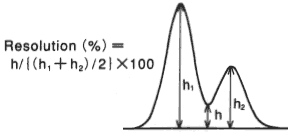
Effect of temperature when analyzing saccharides using SUGAR SC1011 (a column for saccharides analysis) is shown here. Saccharides having carbonyl or aldehyde group may have anomers. It is possible to prevent anomer separation by analyzing at elavated temperature. It is also possible to have higher theoretical plate number and resolution by it. Analyzing at elevated temperature around 80 deg-C is recommended when using columns such as SP0810, SC1011, SC1821, KS-801 and KS-802.
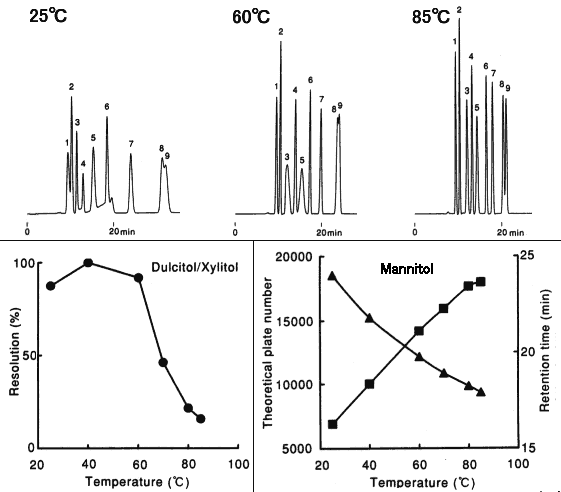

Sample : 0.5%each, 5micro-L
1. Isomaltotriose
2. Sucrose
3. Glucose
4. Lactitol
5. Fructose
6. meso-Erythritol
7. Mannitol
8. Dulcitol
9. Xylitol
Column : Shodex SUGAR SC1011 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI
Raffinose was separated using SUGAR SH1011. Since raffinose can be easily hydrolyzed by acids, it should be analyzed at ordinary temperature. The situation is the same for other saccharides such as sucrose.
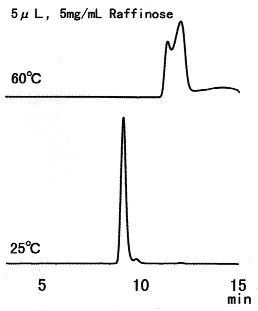
Sample : Raffinose
Column : Shodex SUGAR SH1011 (8.0mmID*300mm) Eluent : 0.005M H2SO4 aq. Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : (above); 60deg-C, (below); 25deg-C
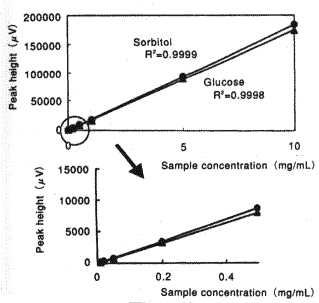
Sample : 20micro-L
|
Correlation Coefficient
(0.2 to 200micro-g) |
|
| Glucose | 0.9998 |
| Sorbitol | 0.9999 |
| Fructose | 0.9998 |
| Sucrose | 0.9999 |
| Lactose | 0.9997 |
| Maltose | 0.9998 |
| meso-Erythritol | 0.9999 |
| Xylitol | 0.9999 |
| Mannitol | 0.9999 |
| Maltitol | 0.9997 |
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
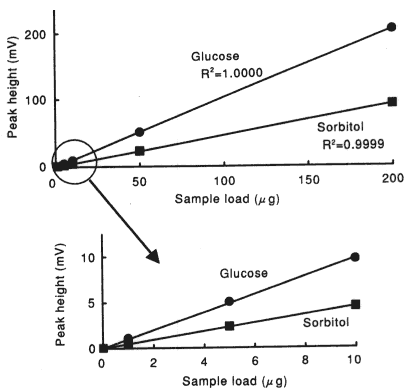
Sample : 10micro-L
|
Correlation Coefficient
(1 to 200micro-g) |
|
| Glucose | 1.0000 |
| Sorbitol | 0.9999 |
| Sucrose | 1.0000 |
| Maltose | 1.0000 |
| Isomaltose | 0.9999 |
| Maltotriose | 1.0000 |
| Raffinose | 1.0000 |
| meso-Erythritol | 1.0000 |
| myo-Inositol | 0.9999 |
| Stevioside | 1.0000 |
Column : Shodex SUGAR SZ5532 (6.0mmID*150mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 60deg-C
Polymer-based Asahipak NH2P-50 4E column has resolved the problem occurring with silica-based amino columns, whose performance falls with the time.
1) Conventional analytical methods used for silica-based amino columns can be applied to the NH2P column.
Eluent —- Mixture of water and acetonitrile which is commonly used for silica-based amino columns can be also used.
Column temperature —- Can be analyzed at room temperature
2) Excellent quantitative analysis can be performed using NH2P column.
3) Further more, the NH2P column can be washed with alkaline solvent.
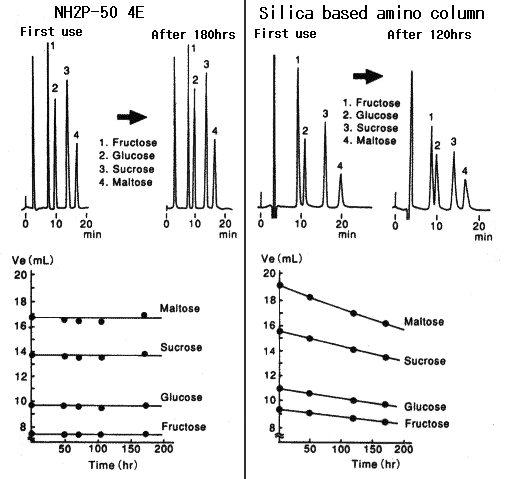
Sample :
1. Fructose
2. Glucose
3. Sucrose
4. Maltose
Columns : Shodex Asahipak NH2P-50 4E, Amino column from other manufacturer (4.6mmID*250mm each) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
Compared with the case when Asahipak NH2P columns are used, when silica-based amino columns are used, detection sensitivity and plate number of saccharides are different according to the type of saccharides. And, the plate number of some specific saccharide such as galactose is extremely low.
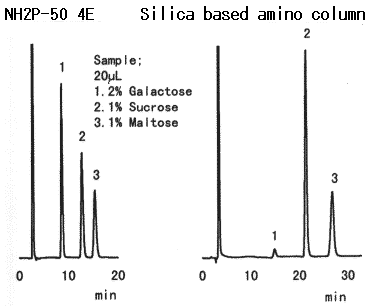
Sample : 20micro-L
1. 2% Galactose
2. 1% Sucrose
3. 1% Maltose
Columns : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm), Silica based amino column (other manufacturer) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
Monosaccharides were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
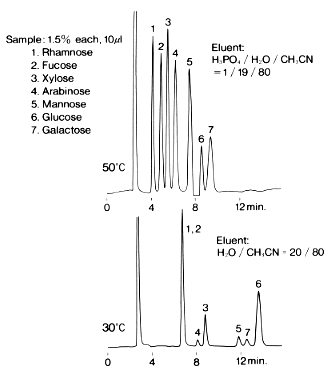
Sample :
1. Rhamnose
2. Fucose
3. Xylose
4. Arabinose
5. Mannose
6. Glucose
7. Galactose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm)
Fluent : (above); H3PO4/H2O/CH3CN=1/19/80
(below); H2O/CH3CN=20/80
Flow rate : 1.0mL/min
Detector : Shodex RI
Column temp. : (above); 50deg-C, (below); 30deg-C
Monosaccharides were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
Sample : 10micro-L
1. 20mg/mL Mannose
2. 20mg/mL Galactose
3. 1mg/mL Glucose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : 10mM *Tetrapropylammonium buffer(pH10.0)/CH3CN=20/80 Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 30deg-C
*How to make Tetrapropylammonium buffer
Please add acetic acid to 10mM Tetrapropylammonium hydroxide and adjust pH10.
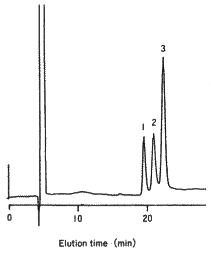
SUGAR SP0810 can effectively separate typical monosaccharides, in particular, lactose and glucose.
Sample :
1. Sucrose
2. Lactose
3. Glucose
4. Lactulose
5. Galactose
6. Fructose
Columns : Shodex SUGAR SP-G (6.0mmID*50mm) + SP0810 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.5mL/min Detector : Shodex RI Column temp. : 80deg-C
Monosaccharides and disaccharides were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
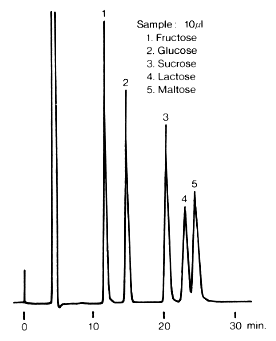
Sample : 5mg/mL each
1. Fructose
2. Glucose
3. Sucrose
4. Lactose
5. Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : H2O/CH3CN=25/75 Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 30deg-C
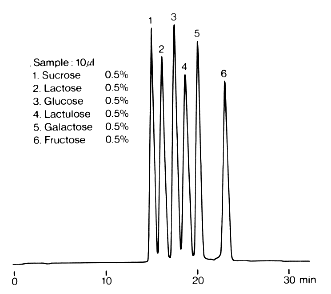
In the Guidelines on Nutrition Labelling in Japan, amino columns of 4.6mmID x 250 mm Length are adopted for the analysis of monosaccharides, disaccharides and sugar alcohols. This chromatogram shows the analysis result of typical monosaccharide and disaccharide standards using an amino column, Asahipak NH2P-50 4E. Since the column is polymer-based, it is durable compared with silica-based columns and can be washed by alkali solution.
The eluent used is the mixture of water and acetonitrile. The elution volume can be changed by changing the mixing ratio.
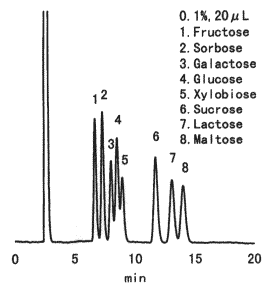
Sample : 0.1% each, 20micro-L
1. Fructose
2. Sorbose
3. Galactose
4. Glucose
5. Xylobiose
6. Sucrose
7. Lactose
8. Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
Asahipak NH2P-50 is a column for the analysis of saccharides by normal phase mode. Under this mode, saccharides are basically eluted in increasing order of molecular weights. An increase of organic solvent in the eluent delays the elution of saccharides. NH2P column allows the separation of saccharides at room temperature without causing anomer separation as these columns are alkaline environment due to the bonded amino groups.
Please compare with the case of RSpak DC-613
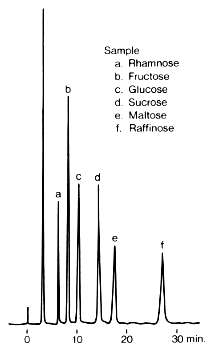
Sample :
a. Rhamnose
b. Fructose
c. Glucose
d. Sucrose
e. Maltose
f. Raffinose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : H2O/CH3CN=25/75 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
RSpak DC-613 isa column for the analysis of saccharides by normal phase mode. Under this mode, saccharides are basically eluted in increasing order of molecular weights. An increase of organic solvent in the eluent delays the elution of saccharides.
For DC-613, it is necessary to do the analysis at 60deg-C for preventing the anomer separation.
Please compare with the case of Asahipak NH2P-50
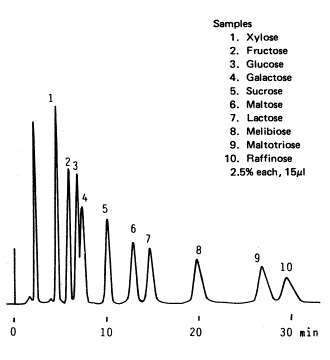
Sample :
1. Xylose
2. Fructose
3. Glucose
4. Galactose
5. Sucrose
6. Maltose
7. Lactose
8. Melibiose
9. Maltotriose
10. Raffinose
Column : Shodex RSpak DC-613 (6.0mmID*150mm) Eluent : H2O/CH3CN=20/80 Flow rate : 1.5mL/min Detector : Shodex RI Column temp. : 70deg-C
The figure shows an example for the analysis of a saccharide mixture contained in an agricultural product after having been harvested and before being processed. The chromatogram has been done with SUGAR SC1011.
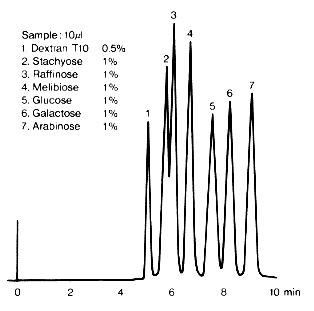
Sample :
1. Dextran T10
2. Stachyose
3. Raffinose
4. Melibiose
5. Glucose
6. Galactose
7. Arabinose
Column : Shodex SUGAR SC1011 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Saccharides typically contained in wood were analyzed using SUGAR SP0810.
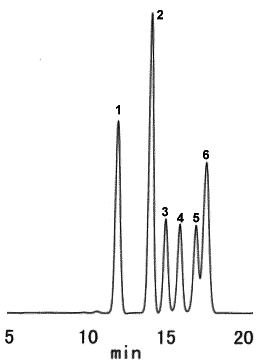
Sample : 5micro-L
1. Cellobiose 1.0%
2. Glucose 1.5%
3. Xylose 0.5%
4. Galactose 0.5%
5. Arabinose 0.5%
6. Mannose 1.0%
Column : Shodex SUGAR SP0810 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 85deg-C
Two sugar analysis columns, SUGAR SP0810, was used to analyze saccharides in wood. Corona charged aerosol detector (CAD) was used as a detector. Comparing to Sacchrides in Wood (1) , the separation of arabinose and mannose was improved by adding CH3CN to the mobile phase.
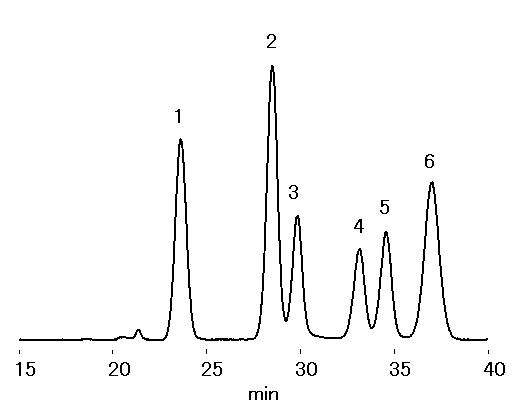
Sample : 10micro-L
1. Cellobiose 1.0%
2. Glucose 1.5%
3. Xylose 0.5%
4. Galactose 0.5%
5. Arabinose 0.5%
6. Mannose 1.0%
Column : Shodex SUGAR SP0810 (8.0mmID*300mm) x 2 Eluent : H2O/CH3CN=77/13 Flow rate : 0.6mL/min Detector : Corona charged aerosol Column temp. : 85deg-C
The figure shows an example for the analysis of palatinose, a synthetic sweetener from glucose and fructose. SUGAR SC1011 is suitable for the separation of saccharides contained in foods generally.
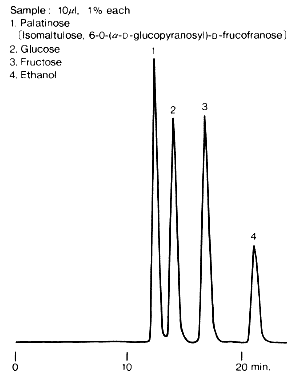
Sample :
1. Palatinose, Isomaltulose
2. Glucose
3. Fructose
4. Ethanol
Columns : Shodex SUGAR SC-LG (6.0mmID*50mm) + SC1011 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 80deg-C
With SUGAR KS-801, high molecular weights and anionic compounds are eluted first, followed by low molecular weight saccharides. Therefore, KS-801 can be used for the analysis of saccharides without any previous desalting of samples such as Worcester sauce, which contains large amounts of sodium chloride and organic acids usually.
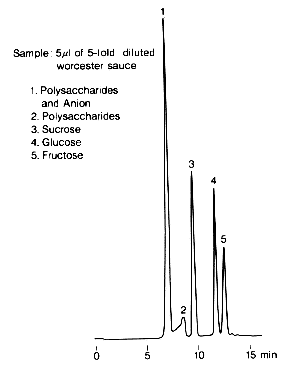
Sample : Worcester sauce
1. Polysaccharides and Anion
2. Polysaccharides
3. Sucrose
4. Glucose
5. Fructose
Column : Shodex SUGAR KS-801 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.7mL/min Detector : Shodex RI Column temp. : 80deg-C
Sugar added yogurt was analyzed using SUGAR SP0810.
(Sample pretreatment)
1) Take 5g of sugar added yogurt in a beaker, add 30mL of pure water and neutralize with 10%(w/v) NaOH.
2) Apply ultrasonic vibration for 30 minutes and make 50 mL solution.
3) Filtrate with No.5B paper filter and then with 0.45 micron membrane filter.
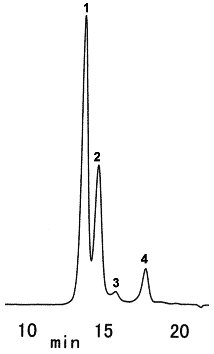
Sample : Yogurt, 5micro-L
1. Sucrose
2. Lactose
3. Glucose
4. Galactose
Column : Shodex SUGAR SP0810 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 85deg-C
When analyzing saccharides in food, it is necessary to remove the substances besides saccharides before analysis. This procedure is called as sample pre-treatment. Here, the analysis result of saccharides in food using Asahipak NH2P-50 4E is shown together with the sample pre-treatment procedure.
(Sample pretreatment)
1) Take 5g of sugar added yogurt in a beaker, add 30mL of pure water and neutralize with 10%(w/v) NaOH.
2) Apply ultrasonic vibration for 30 minutes and make 50 mL solution.
3) Filtrate with No.5B paper filter and then with 0.45 micron membrane filter.
The comparison of NH2P-50 column and silica-based amino column for the analysis of yogurt(sugar added) is as follows:
1)The peaks of fructose and galactose cannot be detected using silica-based amino column.
2)In case of NH2P-50 column, the peak of lactose is lager compared with the case of silica-based amino column. This result shows the fact that most of lactose is adsorbed by silica-based amino column. Since the adsorption of lactose by NH2P-50 column is not so much, the detection limit of NH2P-50 column is lower than that of silica-based amino column.
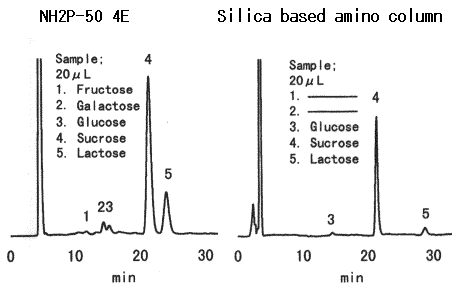
Sample : Yogurt, 20micro-L
1. Fructose
2. Galactose
3. Glucose
4. Sucrose
5. Lactose
Columns : Shodex Asahipak NH2P-50 4E, Amino column from other manufacturer (4.6mmID*250mm each) Eluent : CH3CN/H2O=75/25 Flow rate : 0.6mL/min (NH2P-50), 1.0mL/min (Amino column from other manufacturer) Detector : Shodex RI Column temp. : 25deg-C
When analyzing saccharides in food, it is necessary to remove the substances besides saccharides before the analysis. This procedure is called as sample pre-treatment. Here, the analysis result of saccharides in food using Asahipak NH2P-50 4E is shown together with the sample pre-treatment procedure.
(Sample pre-treatment for roast sweet potato)
1) Make paste of the sample using a food-prcessor.
2) Put 3g of the paste in a beaker and add 30mL of 50%(V/V) ethanol.
3) Extract the substances from the paste using an ultrasonic vibrator for 30 minutes.
4) Put the paste and ethanol in a 50mL messflask and add 50%(V/V) ethanol to make up a 50mL solution.
5) Filtrate the solution with a paper filter(No.5B) to remove the paste.
6) Take a part of filtrated solution and add the same volume of acetonitrile.
7) Pass through a 0.45micron membrane filter before injecting into HPLC.
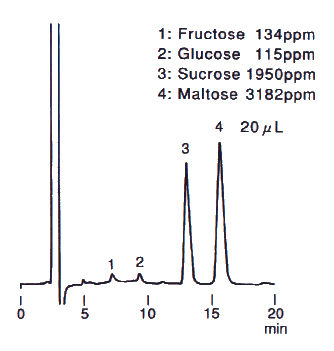
Sample : Sweet potato, roasted, 20micro-L
1. Fructose
2. Glucose
3. Sucrose
4. Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : Room temp.(25deg-C)
When analyzing saccharides in food, it is necessary to remove the substances besides saccharides before the analysis. This procedure is called as sample pretreatment.
Here, the analysis result of saccharides in food using SUGAR SP0810 is shown together with the sample pretreatment procedure.
(Sample pretreatment for roasted sweet potato)
1) Make paste of the sample using a food-prcessor.
2) Put the paste in a beaker and add pure water to make 50-fold solution.
3) Extract the substances from the paste into water using an ultrasonic vibrator for 30 minutes.
4) Filtrate the solution with a paper filter (No.5B) to remove the paste.
5) Pass through a 0.45micron membrane filter before injecting into HPLC.
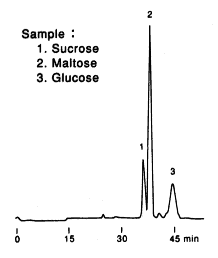
Sample : Sweet potato, roasted
1. Sucrose
2. Maltose
3. Glucose
Columns : Shodex SUGAR SP-G (6.0mmID*50mm) + SP0810 (8.0mmID*300mm) x 2 Eluent : H2O Flow rate : 0.4mL/min Detector : Shodex RI Column temp. : 80deg-C
When analyzing saccharides in food, it is necessary to remove the substances besides saccharides before the analysis. This procedure is called as sample pretreatment.
Here, the analysis result of saccharides in food using SUGAR SP0810 is shown together with the sample pretreatment procedure.
(Sample pretreatment for soybean flour)
1) Put the soybean flour in a beaker and add pure water to make 50-fold solution.
2) Extract the substances from the paste into water using an ultrasonic vibrator for 30 minutes.
3) Perform ultrafiltration (mw : 10,000).
4) Pass through a 0.45micron membrane filter before injecting into HPLC.
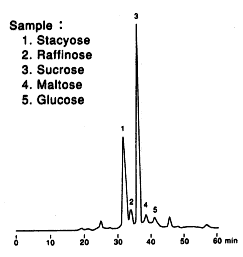
Sample : Soybean Flour
1. Stachyose
2. Raffinose
3. Sucrose
4. Maltose
5. Glucose
Columns : Shodex SUGAR SP-G (6.0mmID*50mm) + SP0810 (8.0mmID*300mm) x 2 Eluent : H2O Flow rate : 0.4mL/min Detector : Shodex RI Column temp. : 80deg-C
When analyzing saccharides in food, it is necessary to remove the substances besides saccharides before the analysis. This procedure is called as sample pre-treatment. Here, the analysis result of saccharides in food using Asahipak NH2P-50 4E is shown together with the sample pre-treatment procedure.
(Sample pre-treatment for milk coffee)
1) Put 5g of the sample in a 10mL messflask.
2) Add ultra pure water to make up 10mL solution.
3) Perform ultrafiltration (mw:10,000).
4) Take a part of the sample solution and add the same volume of acetonitrile.
5) Pass through a 0.45micron membrane filter before injecting into HPLC.
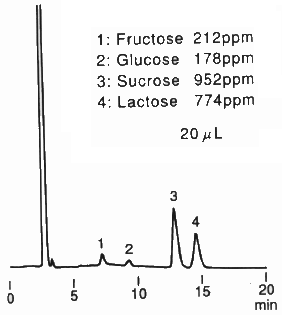
Sample : Milk coffee, 20micro-L
1. Fructose
2. Glucose
3. Sucrose
4. Lactose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : Room temp.(25deg-C)
When analyzing saccharides in food, it is necessary to remove the substances besides saccharides before the analysis. This procedure is called as sample pre-treatment. Here, the analysis result of saccharides in food using Asahipak NH2P-50 4E is shown together with the sample pre-treatment procedure.
(Sample pre-treatment for jelly)
1) Put 5g of jelly in a beaker and add 30mL of 50%(V/V) ethanol.
2) Extract the substances using an ultrasonic vibrator for 30 minutes.
3) Put the paste and ethanol in a 50mL messflask and add 50%(V/V) ethanol to make up a 50mL solution.
4) Filtrate the solution with a paper filter(No.5B).
5) Take a part of filtrated solution and add the same volume of acetonitrile.
6) Pass through a 0.45micron membrane filter before injecting into HPLC.
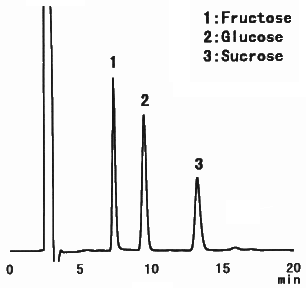
Sample : Jelly, 20micro-L
1. Fructose
2. Glucose
3. Sucrose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : Room temp.(25deg-C)
Saccharides are extracted from bread using water and three saccharides, lactose, glucose and fructose are separated using SUGAR KS-801 (a column for saccharides analysis). There is a unknown peak after the peak of fructose. It is recommended to use a guard column for the analysis of actual sample which may contain impurities.
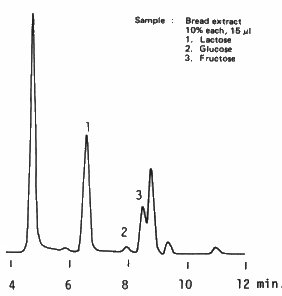
Sample : Bread
1. Lactose
2. Glucose
3. Fructose
Columns : Shodex SUGAR KS-G (6.0mmID*50mm) + KS-801 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Raspberry jam was analyzed. It was found that lots of oligosaccharides are contained in the sample. Two columns, SUGAR SC1011 and SC1821 were used and better separation was obtained by SC1821 because of its larger pore size.
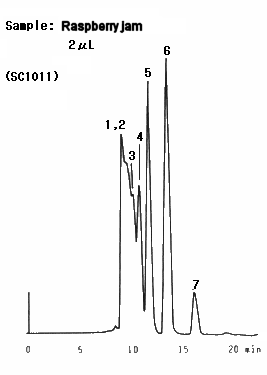
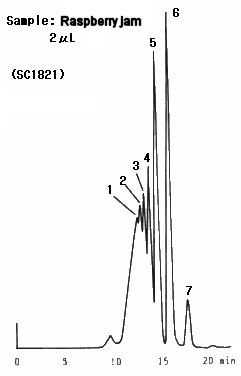
Sample : Raspberry jam
1. Oligosaccharide(DP-6)
2. Oligosaccharide(DP-5)
3. Oligosaccharide(DP-4)
4. Oligosaccharide(DP-3)
5. Maltose
6. Glucose
7. Fruct ose
Columns : (left); Shodex SUGAR SC1011, (right); SC1821 (8.0mmID*300mm each) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 80deg-C
Saccharides in candy were analyzed using SUGAR SC1011 and SC1821 (columns for saccharides analysis). In the sample, rather small quantity of oligosaccharides (larger than trisaccharide) were contained. The main component is maltose. Comparison of two columns, SC1011 and SC1821 shows that better separation is obtained by SC1821 because of its larger pore size.
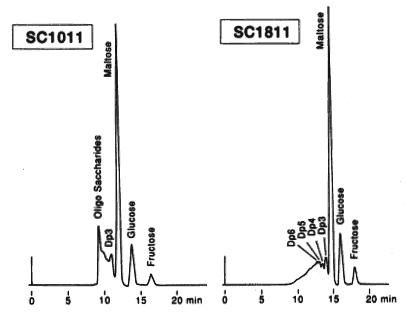
Sample : Candy solution, 5micro-L
Oligosaccharide : DP-6, DP-5, DP-4, DP-3
Maltose
Glucose
Fructose
Columns : Shodex SUGAR SC1011, SC1821 (8.0mmID*300mm each) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 80deg-C
Saccharides in lactic acid beverage and fermented milk were analyzed using SUGAR SH1011 (a column for saccharides analysis). The main components of lactic acid beverage were glucose and fructose and small quantity of lactic acid was also observed. The main components of fermented milk was glucose and small quantity of lactic acid and acetic acid were also observed.
(Sample preparation)
The samples were diluted with water and sulfosalicylic acid was added to eliminate proteins.
Then they were separated using a centrifugal machine and the top clear layers were took and filtrated using 25 micron disposable filter.
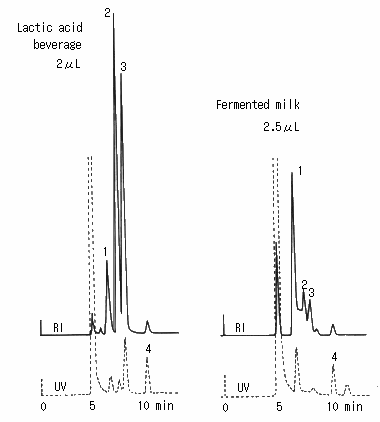
Sample :
Lactic acid beverage and fermented Milk
1. Lactose
2. Glucose
3. Fructose
4. Lactic acid
Columns : Shodex SUGAR SH-G (6.0mmID*50mm) + SH1011 (8.0mmID*300mm) Eluent : 5mM H2SO4 aq. Flow rate : 1.0mL/min Detector : Shodex RI, UV(210nm) Column temp. : 50deg-C
Saccharides in orange and melon were analyzed using SUGAR SH1011. Besides saccharides, some unknown peaks were observed though organic acids and pigments were eliminated by the mini-column.
(the pretreatment of fruit juice)
First, the sample was filtrated using a 25 micron disposable filter.
Then, it was treated with a mini-column packed with anion exchanger. And, it was filtrated again.
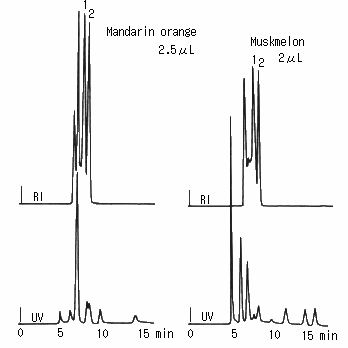
Sample :
Mandarin orange, 2.5micro-L
Musk melon, 2micro-L
1. Glucose
2. Fructose
Columns : Shodex SUGAR SH-G (6.0mmID*50mm) + SH1011 (8.0mmID*300mm) Eluent : 5mM H2SO4 aq. Flow rate : 1.0mL/min Detector : Shodex RI, UV(210nm) Column temp. : 50deg-C
Saccharides in orange juice were analyzed using SUGAR SC1011 (a column for saccharides analysis). For the pretreatment of juice, first, the sample was filtrated 2 times using a 0.45 micron disposal filter. Then, it was treated with a mini-column packed with anion ion exchanger. And it was filtered again. In front of glucose and fructose peaks, a large peak was observed and it was considered to be oligosaccharides.
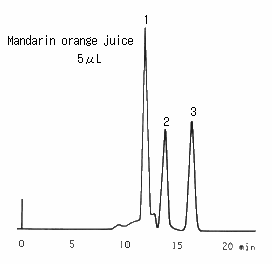
Saccharides in orange juice were analyzed using SUGAR SC1011 (a column for saccharides analysis). For the pretreatment of juice, first, the sample was filtrated 2 times using a 0.45 micron disposal filter. Then, it was treated with a mini-column packed with anion ion exchanger. And it was filtered again. In front of glucose and fructose peaks, a large peak was observed and it was considered to be oligosaccharides.
(Sample pretreatment)
1) Take 5g of chocolate cake in a vial for centrifuge.
2) Add 30 mL of petroleum ether and stir it for 15 minutes.
3) Then use centrifuge for 10 minutes at 2,000 rpm and take off the supernatant.
4) Heat the sample solution to remove petroleum ether in the water bath at 40deg-C.
5) Add 30 mL of the mixture of 50%(V/V) ethanol solution.
6) Apply ultrasonic vibration for 30 minutes and make 50 mL of solution with the mixture of 50%(V/V) ethanol.
7) Filtrate with No.5B paper filter.
8) Take 3mL of filtrated sample and add 3mL of acetonitrile.
9) Filtrate with 0.45 micron membrane filter.
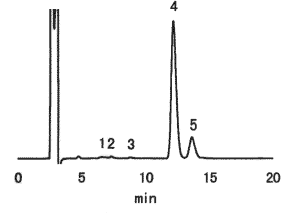
Sample : Chocolate cake
| 20micro-L injection |
Concentration (mg/mL) |
Contents(g) in 100g |
| 1.Fructose | 0.12 | 0.2 |
| 2.Sorbitol | 0.20 | 0.4 |
| 3.Glucose | 0.12 | 0.2 |
| 4.Sucrose | 17.84 | 35.7 |
| 5.Lactose | 3.70 | 7.4 |
| 6.Maltose | 0.14 | 0.3 |
| Total | 44.2 |
Indicated value by Producer
| Components in 100g | |||
|---|---|---|---|
| Calorie | 557kcal | Saccharides | 52.8g |
| Proteins | 8.3g | Sodium | 75mg |
| Lipids | 34.8g | ||
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
Saccharides in chocolate cake were analyzed using SUGAR SZ5532 (a column for saccharides analysis).
(Sample pretreatment)
1) Take 5g of chocolate cake in a vial for centrifuge, add 40 mL of petroleum ether and stir it for 15 minutes. Then use centrifuge for 10 minutes at 2,000 rpm and take off the supernatant.
2) Again, add 40 mL of petroleum ether and stir it for 15 minutes and take off remaining petroleum ether completely in a water bath of 40 deg-C.
3) Add 30 mL of pure water, apply ultrasonic vbvration for 30 minutes and make 50 mL of solution with pure water.
4) Filtrate with No.5B paper filter and ultrafiltrate at Mw 10,000.
5) Take a part of filtrated sample and add same quantity of acetonitrile.
6) Filtrate with 0.45 micron membrane filter.
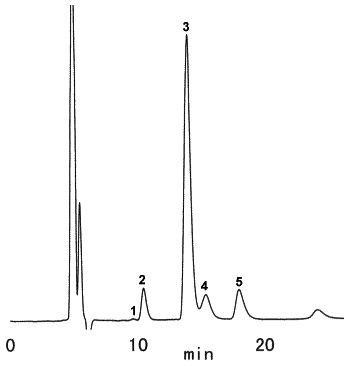
Sample : Chocolate cake, 10micro-L
1. Fructose
2. Glucose
3. Sucrose
4. Maltose
5. Lactose
Column : Shodex SUGAR SZ5532 (6.0mmID*150mm) Eluent : CH3CN/H2O=75/25 Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 60deg-C
SUGAR SZ5532 is a column, which separates saccharides by ligand exchange and normal phase modes. Basically saccharides are eluted in order of increasing molecular weights. This column allows a base-line separation of maltose and isomaltose, which could not be separated with other SUGAR series.
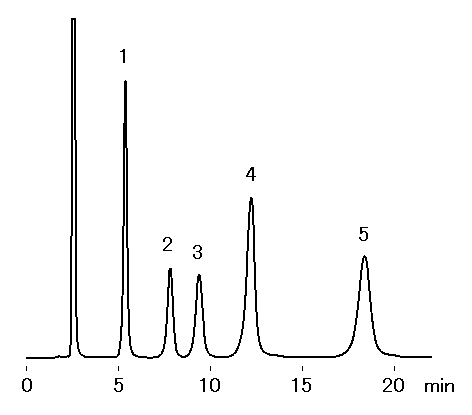
Sample : 0.5% each, 20micro-L
1. Glucose
2. Maltose
3. Isomaltose
4. Maltotriose
5. Isomaltotriose
Column : Shodex SUGAR SZ5532 (6.0mmID*150mm) Eluent : H2O/CH3CN=25/75 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 60deg-C
High sensitive analysis of saccharides was performed using Asahipak NH2P-50 2D.
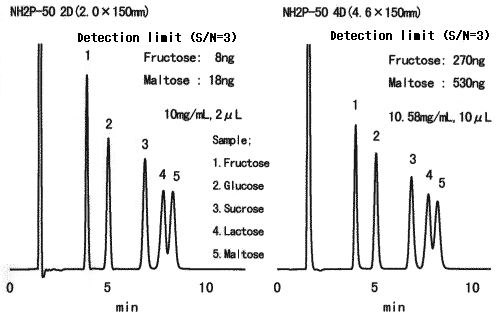
Sample :
1. Fructose
2. Glucose
3. Sucrose
4. Lactose
5. Maltose
Columns : Shodex Asahipak NH2P-50 2D Shodex Asahipak NH2P-50 4D
(2mmID*150mm) (4.6mmID*150mm)
Eluent : CH3CN/H2O=75/25 CH3CN/H2O=75/25
Flow rate : 0.2mL/min 1.06mL/min
Detector : Shodex RI(small cell volume) Shodex RI(conventional type)
Column temp. : 25deg-C 25deg-C
High sensivity analysis of saccharides in powdered milk was performed using Asahipak NH2P-50 4E (a column for saccharides analysis) and an electrochmical detection system.
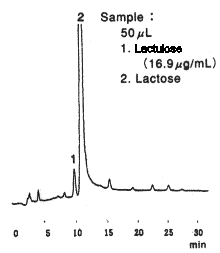
Sample : powdered Milk
1. Lactulose
2. Lactose
Instrument : Agilent 1050 system
Reagent : 0.6M LiOH 0.8mL/min
Mode : pulse
Working electrode : Gold
Potential : Pot 1=0.65V, Pot 2=-0.95V, Pot=0.15V
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm)
Eluent : (A); CH3CN/H2O=80/20
(B); H2O
0min to 40min, 5% (B) to 40% (B)
40min to 45min, 40% (B)
Flow rate : 1.0mL/min
Detector : Agilent 1049A Electrochemical
Column temp. : 40deg-C
Glucose derivatives were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
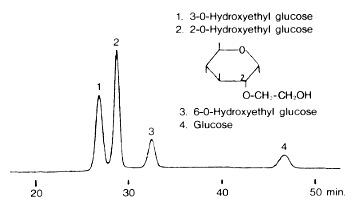
Sample :
1. 3-O-Hydroxyethyl glucose
2. 2-O-Hydroxyethyl glucose
3. 6-O-Hydroxyethyl glucose
4. Glucose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : H2O/CH3CN=15/85 Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 30deg-C
Extract of wheat rod were analyzed using Asahipak NH2P-50 (a column for saccharides analysis).
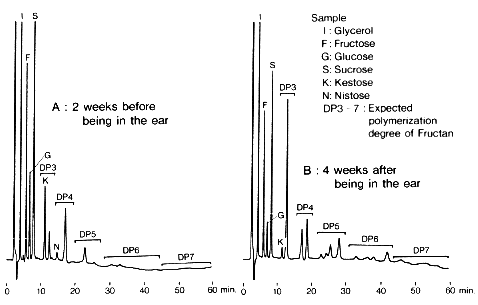
Sample :
I. Glycerol
F. Fructose
G. Glucose
S. Sucrose
K. Kestose
N. Nystose
DP3-7. Expected polymerization degree of Fructan
Columns : Shodex Asahipak NH2P-50G 4A (4.6mmID*10mm) + NH2P-50 4E (4.6mmID*250mm) Eluent : 50mM Ammonium acetate/CH3CN=30/70 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 40deg-C
Courtesy of Dr. Tsuchiya, Ibaraki University
Effect of organic solvent in eluent when saccharides are analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis) is shown.
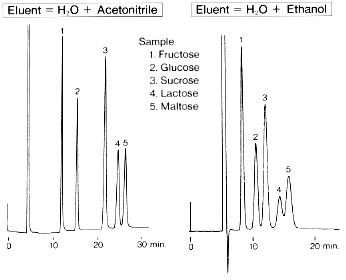
Sample :
1. Fructose
2. Glucose
3. Sucrose
4. Lactose
5. Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm)
Eluent : (left); H2O/CH3CN=25/75
(right); H2O/C2H5OH=10/90
Flow rate : 0.6mL/min
Detector : Shodex RI
Column temp. : 30deg-C
Saccharides which are usually contained in foods were separated by SUGAR SC1011 (a column for saccharides analysis).
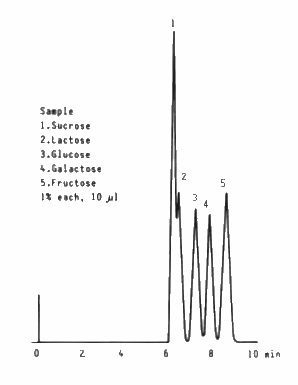
Sample : 1% each, 10micro-L
1. Sucrose
2. Lactose
3. Glucose
4. Galactose
5. Fructose
Column : Shodex SUGAR SC1011 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Canned soft drink containing coffee and sugar, tea and sugar and orange juice were analyzed using LC/MS. Stable baselines were obtained by using a polymer-based amino column, Asahipak NH2P-50 2D.
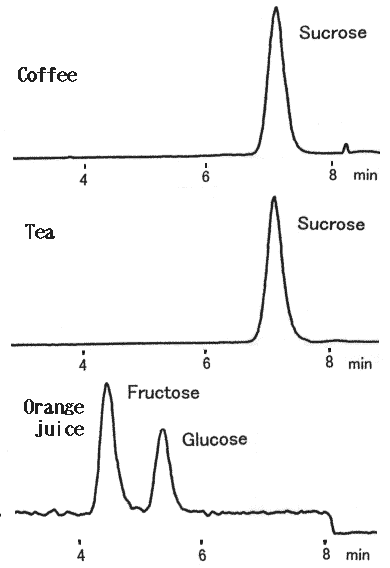
Sample : Soft drink
Canned coffee with sugar
1. Sucrose(m/z=378)
Canned tea with sugar
1. Sucrose(m/z=378)
Canned orange juice
1. Fructose(m/z=216)
2. Glucose(m/z=216)
(HPLC) Instrument : Agilent 1100 Column : Shodex Asahipak NH2P-50 2D (2.0mmID*150mm) Eluent : H2O/CH3CN=25/75 Flow rate : 0.2mL/min Column temp. : 40deg-C (Post column) Reagent : CH3CN/CHCl3=50/50 Flow rate : 0.2mL/min (MS) Instrument : Agilent 1100MSD Mass range : 100-500(m/z) Ionization : APCl Mode : SIM(M+Cl) Polarity : Negative Fragmentor : 20V Nebulizer : N2(40psi) Drying gas : N2(10L/min, 350deg-C) Corona current : 30micro-A Vaporizer temp. : 400deg-C
Monosaccharides which compose sugar chain are separated using SUGAR SH1011. Glycoproteins and glycolipids, which are exist in animals and palnts, are composed of protein and lipid molecles combined with sugar chains. And, sugar chains are composed of monosaccharides such as D-glucose, D-mannose, L-fucose, N-acetylglucosamine, N-acetylgalactosamine and sialic acids. N-Acetylneuraminic acid is the most popular sialic acid.
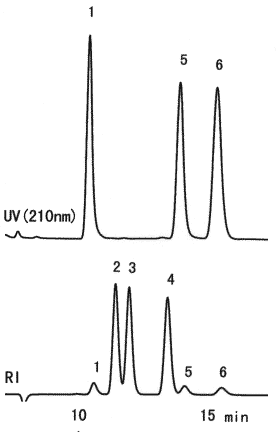
Sample :
1. N-Acetylneuraminic acid, Sialic acid
2. Glucose
3. Mannose
4. Fucose
5. N-Acetyl-D-glucosamine
6. N-Acetyl-D-galactosamine
Column : Shodex SUGAR SH1011 (8.0mmID*300mm)
Eluent : 0.005M H2SO4 aq.
Flow rate : 0.6mL/min
Detector : (above); UV(210nm)
(below); Shodex RI
Column temp. : 60deg-C
Asahipak NH2P-50 4E (a column for saccharides analysis) allows separation of Maltose and Nigerose, which could not be separated with other SUGAR series.
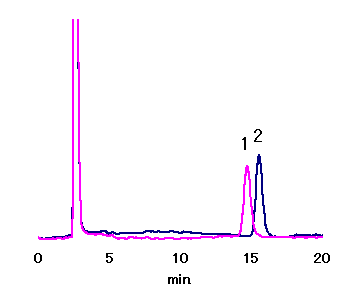
Sample : 20micro-L
1. 1.25mg/mL Nigerose
2. 1.25mg/mL Maltose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=75/25 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
SUGAR SH1821 column was used to separate malto-oligosaccharides, glucose, lactic acid, glycerol, acetic acid, methanol and ethanol simultaneously in one single run. The separation occurred based on size exclusion, ion exclusion, and reversed phase modes. With the real-time monitoring of fermentation process, appropriate adjustments can be made to maximize the ethanol yield. The retention of organic acids can be controlled by changing the concentration of sulfuric acid in the eluent. It is easy to obtain fast analysis or high resolution data simply by varying the flow rate. The use of a guard column SH-G prolongs the SH1821 column life.
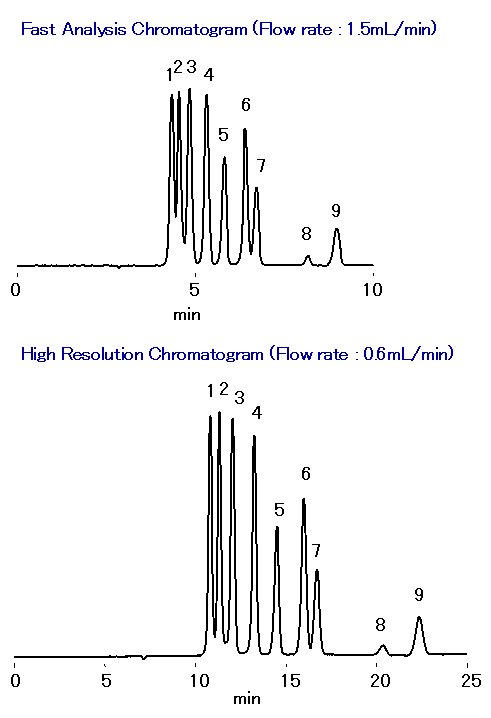
Sample : 0.05% each, 5micro-L
1. Maltotetraose
2. Maltotriose
3. Maltose
4. Glucose
5. Lactic acid
6. Glycerol, Glycerin
7. Acetic acid
8. Methanol, Methyl alcohol
9. Ethanol, Ethyl alcohol
Column : Shodex SUGAR SH1821 (8.0mmID*300mm) Eluent : 0.5mM H2SO4 aq. Flow rate : 1.5mL/min or 0.6mL/min Detector : Shodex RI Column temp. : 75deg-C
Galactinol is a conjugate of myo-inositol and galactose. Furthermore, galactinol and sucrose are transformed into raffinose through the action of raffinose synthase. In this application, the SUGAR SC1011 column was used to analyze the 5 types of sugars and sugar alcohols involved in these reactions.
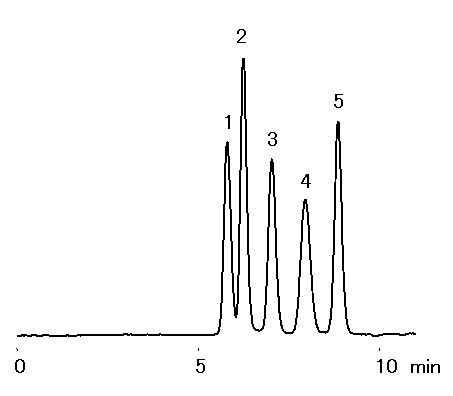
Sample : 20micro-L, 0.1% each
1. Raffinose
2. Sucrose
3. Galactinol
4. Galactose
5. myo-Inositol
Column : Shodex SUGAR SC1011 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Rare sugars are known to carry out various biological functions and are used in a research setting for medical, food, and cosmetics applications. In this application, rare sugars psicose and allose were simultaneously analyzed with the SUGAR SP0810 saccharide analysis column.
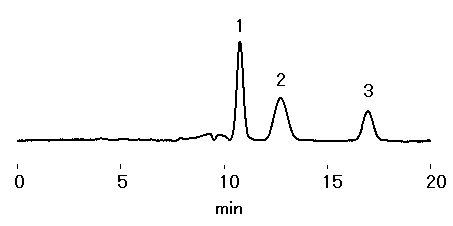
Sample : 0.025% each, 10micro-L
1. Fructose
2. Allose
3. Psicose
Column : Shodex SUGAR SP0810 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
RSpak DC-613 column is for the separation of sugars using ligand-exchange and normal-phase modes. The ligand used is Na, thus even for the samples containing sodium salt does not require desalting sample preparation steps. The samples treated with acids can be analyzed after neutralizing with sodium hydroxide.
*DC-613 sugar analysis requires mobile phase with high concentration of ACN, thus samples containing a large amount of salt may cause precipitation.
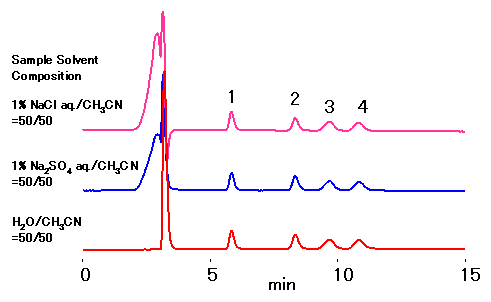
Sample : 10micro-L, 0.1% each
1. Xylose
2. Sucrose
3. Cellobiose
4. Lactose
Column : Shodex RSpak DC-613 (6.0mmID*150mm) Eluent : CH3CN/H2O=70/30 Flow rate : 0.8mL/min Detector : Shodex RI Column temp. : 50deg-C
Mono- and di-saccharides in ionic liquid were analyzed by RSpak DC-613. Saccharides were separated under ligand-exchange and HILIC separation modes. The presence of one type of ionic liquids, 1-Ethyl-3-methylimidazolium chloride, did not interfere with saccharide separation.
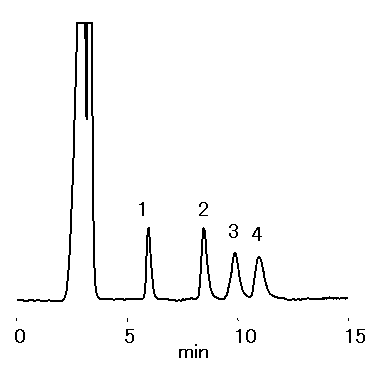
Sample : 0.1% each, 10micro-L
1. Xylose
2. Sucrose
3. Cellobiose
4. Lactose
※Sample solution :
1% 1-Ethyl-3-methylimidazolium Chloride/CH3CN=50/50
Column : Shodex RSpak DC-613 (6.0mmID*150mm) Eluent : CH3CN/H2O=70/30 Flow rate : 0.8mL/min Detector : Shodex RI Column temp. : 50deg-C
Meglumine was analyzed by multi-mode column, RSpakNN-414. Meglumine is an amino-sugar derived from sorbitol. It is often used in pharmaceuticals or radio contrast media.
*To stabilize the elution time, inject formic acid (diluted by water 1+9) before the analysis.
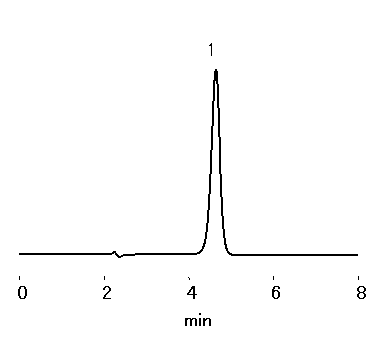
Sample : 10micro-L
1. Meglumine 0.1%
Column : Shodex RSpak NN-414 (4.6mmID*150mm) Eluent : Trifluoroacetic acid/Formic acid/H2O=0.05/0.3/100 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 35deg-C
Two heptulose, mannoheptulose and sedoheptulose, were separated by sugar column, SUGAR KS-801.
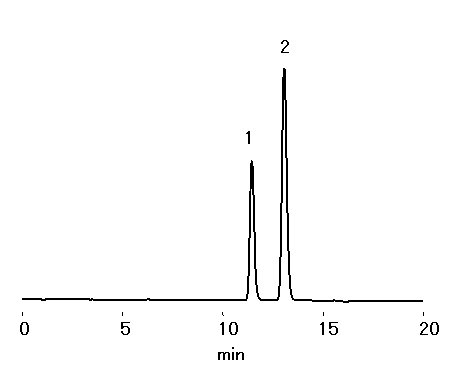
Sample : 0.1% each, 10micro-L
1. Mannoheptulose
2. Sedoheptulose
Column : Shodex SUGAR KS-801 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 80deg-C
Since corona charged aerosol detector (CAD) measures components of elute as well as the analyte, anything elute from column influences the baseline largely. The polymer-based amino column, Asahipak NH2P-50 4E has very small “bleeding” and thus provides stable baseline without much noise compared to silica-based columns.
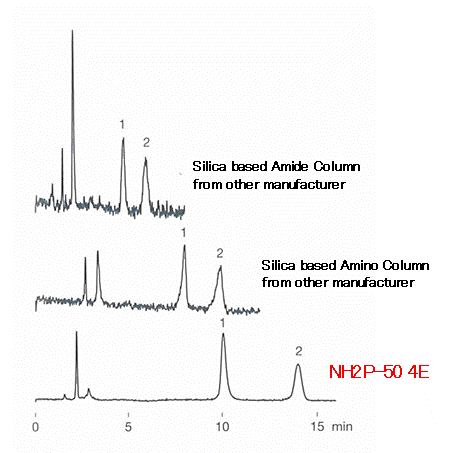
Sample : 40micro-g/mL each, 5micro-L
1. Fructose
2. Glucose
Columns : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm)
Silica based amino column from other manufacturer (4.6mmID*250mm)
Silica based amide column from other manufacturer (4.6mmID*150mm)
Eluent : H2O/CH3CN=20/80
Flow rate : 1.0mL/min
Detector : Corona charged aerosol
Column temp. : 30deg-C (Amino column), 80deg-C (Amide column)
Asahipak NH2P-40 3E is filled with smaller-sized gel, 4 micro-m, and packed in a 3.0 mm internal diameter (I.D.) column housing. The selected ID size allows the column to be used with a conventional LC system, i.e., without a need of semi-micro or any other special LC system. NH2P-40 3E provides higher resolution compared to Asahipak NH2P-50 4E. NH2P series is one of the best fits for the saccharides analysis using HILIC mode. The solvent required for NH2P-40 3E column is approximately 1/3 of the NH2P-50 4E column.
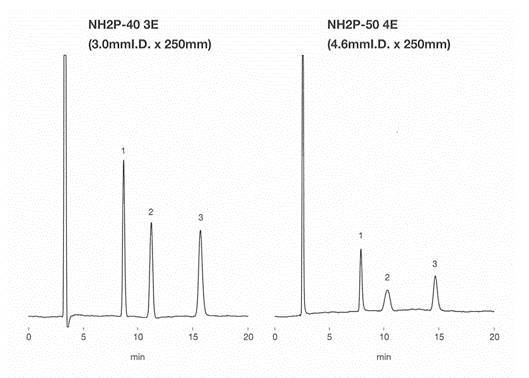
Sample : 0.5% each, 4micro-L
1. Fructose
2. Glucose
3. Sucrose
Data obtained by a conventional RI (0.25 mm ID tubing).
| NH2P-40 3E | NH2P-50 4E | ||
| TPN (Sucrose) | 11,300 | 8,700 | |
| Rs | Fructose/Glucose | 6.4 | 4.2 |
| Glucose/Sucrose | 8.5 | 5.9 | |
Columns : Shodex Asahipak NH2P-40 3E (3.0mmID*250mm), NH2P-50 4E (4.6mmID*250mm) Eluent : H2O/CH3CN=25/75 Flow rate : (NH2P-40 3E) 0.35mL/min, (NH2P-50 4E) 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
SUGAR SZ5532 (a column for saccharides analysis) can effectively separate Sucrose and its isomer Turanose.
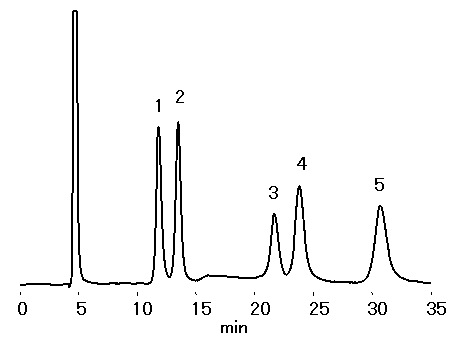
Sample : 0.5% each, 10micro-L
1. Fructose
2. Glucose
3. Sucrose
4. Turanose
5. Lactose
Column : Shodex SUGAR SZ5532 (6.0mmID*150mm) Eluent : CH3CN/H2O=80/20 Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 60deg-C
Saccharides and furfurals were analyzed simultaneously using SUGAR KS-801. KS-801 works under ligand exchange and size exclusion mode. The separation can be achieved using only water as a mobile phase.
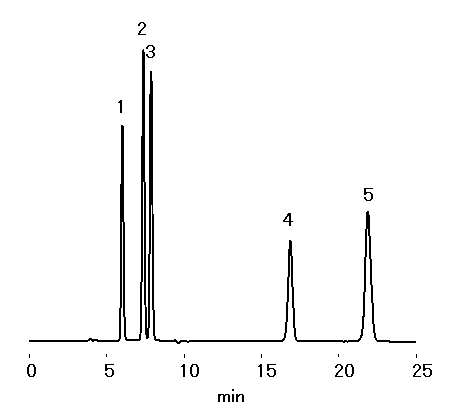
Sample : 0.1% each, 10micro-L
1. Sucrose
2. Glucose
3. Fructose
4. 5-HMF
5. Furfural
Column : Shodex SUGAR KS-801 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
SUGAR KS-801 is packed with styrene divinylbenzene copolymer base gel, modified with sulfonic acid sodium salt. KS-801 is suitable for the trehalose hydrate analysis indicated in 16th edition of Japanese Pharmacopeia.
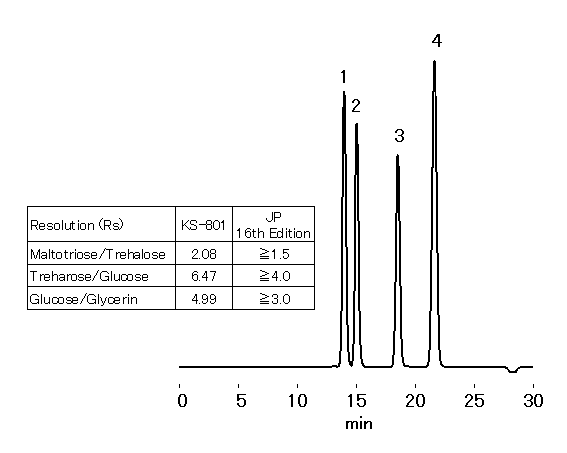
Sample : 20micro-L
1. Maltotriose 0.5%
2. Trehalose 0.5%
3. Glucose 0.5%
4. Glycerin 1.0%
Column : Shodex SUGAR KS-801 (8.0mmID*300mm) Eluent : H2O Flow rate : 0.4mL/min Detector : Shodex RI Column temp. : 80deg-C