Oligosaccharides
For sample containing both NaCl and anionic compounds such as organic acids, SUGAR KS-802 is best suitable for the analysis. As anionic compounds are eluted first by ion exclusion mode, the column allows the analysis of oligosaccharides up to 10mers without any previous desalting.
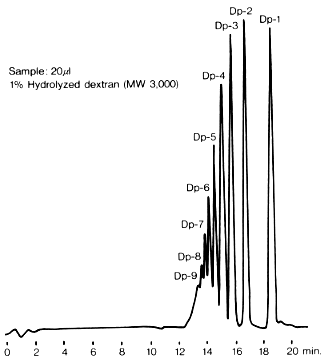
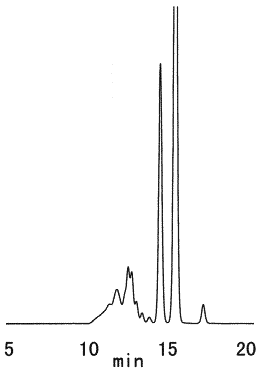
Sample : Dextran,Hydrolyzed dextran
Columns : Shodex SUGAR KS-802 (8.0mmID*300mm) x 2 Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Asahipak NH2P-50 4E is used for the separation of hydorolyzed dextran, and two methods, isocratic elution and gradient elution are compared. Using gradient elution, better separation can be obtained. Until recently, gradient elution could not be used for the separation of sugars because there was no detector to be used. ELSD (evaporative light scattering detector) solved this problem and enables the separtion of sugars using gradient elution.
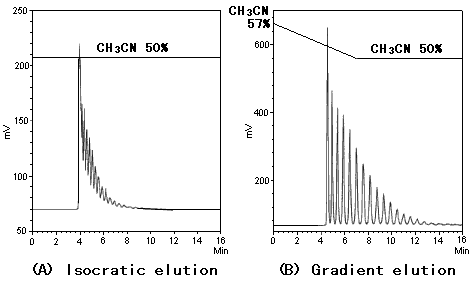
Sample : 0.5% Hydrolyzed Dextran (Dextran)
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm)
Eluent : (A); CH3CN/H2O=50/50
: (B); Linear gradient:
0min to 7 min, CH3CN/H2O=57/43 to 50/50
7min to 16 min, CH3CN/H2O=50/50
Flow rate : 1.0mL/min
Detector : ELSD
Column temp. : 30deg-C
The change of hyrolyzed dextran is analyzed. The analysis was done using SUGAR KS-802.
(Sample preparation)
1) Hydrolyze 4% dextran aquaous solution using 1M HCl at 100 deg-C.
2) Take sample before and after 30, 40 and 50min from the start of the hydrolyzation.
3) Neutralize the sample adding cold 1M NaOH aaquaous solution.
4) Desalt the sample passing it through ion-exchange resin.
5) Filtrate with 0.45 micron membrane filter.
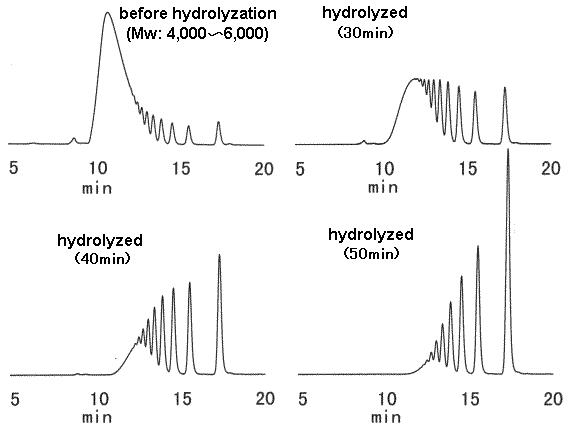
Sample : Hydrolyzed dextran, 20micro-L (Dextran)
Columns : Shodex SUGAR KS-802(8.0mmID*300mm) x 2 Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Isomaltoologosaccharides were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
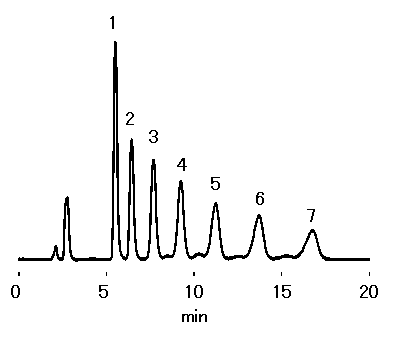
Sample : Isomaltooligosaccharides, 10micro-L
1. Glucose
2. Isomaltose
3. Isomaltotriose
4. Isomaltotetraose
5. Isomaltopentaose
6. Isomaltohexaose
7. Isomaltoheptaose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=60/40 Flow rate : 0.8mL/min Detector : Shodex RI Column temp. : 30deg-C
Maltooligosaccharides were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
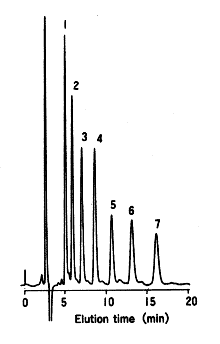
Sample : Maltooligosaccharides 5mg/mL each, 10micro-L
1. Glucose
2. Maltose
3. Maltotriose
4. Maltotetraose
5. Maltopentaose
6. Maltohexaose
7. Maltoheptaose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=60/40 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 30deg-C
Chitooligosaccharides were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
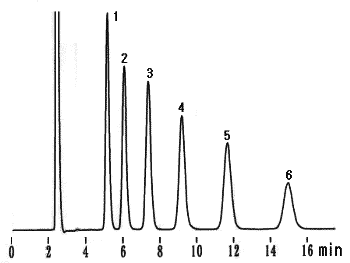
Sample : 2% Mixture of N-Acetyl-chitooligosaccharides, 20micro-L
(Seikagaku Co. code:800104 Chitooligosaccharides Mixture)
1. N-Acetyl-D-glucosamine
2. di-N-Acetyl-chitobiose
3. tri-N-Acetyl-chitotriose
4. tetra-N-Acetyl-chitotetraose
5. penta-N-Acetyl-chitopentaose
6. hexa-N-Acetyl-chitohexaose
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=70/30 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
Chitosan-oligosaccharides are oligosaccharides which have the structure that D-glucosamines are bonded by beta-1,4 bonding and physiologically acitive. From dimer to hexamer of chitosan-oligosaccharide are separated using Asahipak NH2P-50 4E, a column for sugar analysis and evaporative light scattering detector (ELSD).
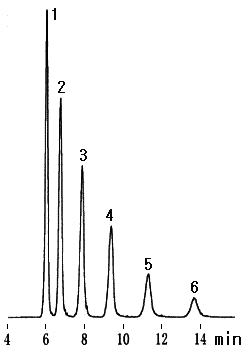
Sample : Mixture of Chitosan-oligosaccharides (Chitooligosaccharides)
(Seikagaku Co. code:800105 Chitosan-Oligosaccharides)
1. D-Glucosamine
2. Chitobiose: dimer
3. Chitotriose: trimer
4. Chitotetraose: tetramer
5. Chitopentaose: pentamer
6. Chitohexaose: hexamer
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=70/30 Flow rate : 1.0mL/min Detector : ELSD Column temp. : 30deg-C
Chitosan-oligosaccharides were analyzed using Asahipak NH2P-50 4E (a column for saccharides analysis).
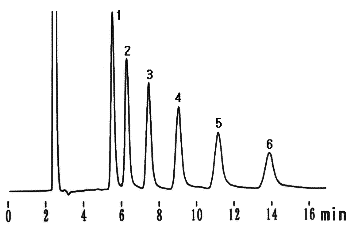
Sample : 2% Mixture of Chitosan-oligosaccharides (Chitooligosaccharides), 20micor-L
(Seikagaku Co. code: 800105 Chitosan-Oligosaccharides)
1. D-Glucosamine
2. Chitobiose hydrochloride
3. Chitotriose hydrochloride
4. Chitotetraose hydrochloride
5. Chitopentaose hydrochloride
6. Chitohexaose hydrochloride
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=70/30 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
Cyclodextrins have the structure that D-glucoses are bonded cyclically by alpha-1,4 bonding. Cyclodexrins inculde organics and change their characteristics. Therefore, cyclodextrins are used as stabilizers, antioxidants, antivolatilizers and food additives. Three kinds of cyclodextrins which consist of 6 glucoses(alpha), 7 glucoses(beta) and 8 glucoses(gamma) are analyzed. Two columns, Asahipak GS-220 HQ and SUGAR KS-802 are used and the cromatograms show that better peak shapes can be obtained using GS-220 HQ.
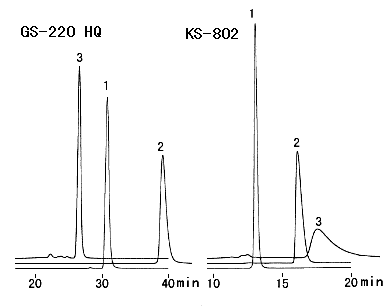
Sample :
1. alpha-Cyclodextrin
2. beta-Cyclodextrin
3. gamma-Cyclodextrin
Columns : Shodex Asahipak GS-220 HQ (7.5mmID*300mm) x 2 Shodex SUGAR KS-802 (8.0mmID*300mm) x 2 Eluent : H2O H2O Flow rate : 0.6mL/min 1.0mL/min Detector : Shodex RI Shodex RI Column temp. : 60deg-C 80deg-C
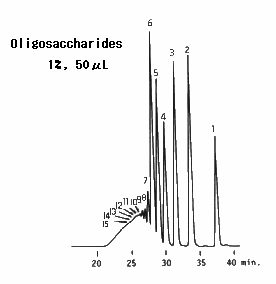
Oligosaccharides and sugar alcohols were analyzed using SUGAR SC1821 (a column for saccharides analysis).
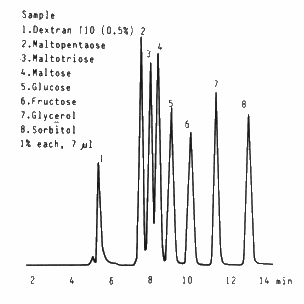
Sample : 7micro-L
1. 0.5% Dextran T10
2. 1.0% Maltoheptaose
3. 1.0% Maltotriose
4. 1.0% Maltose
5. 1.0% Glucose
6. 1.0% Fructose
7. 1.0% Glycerol
8. 1.0% Sorbitol
Column : Shodex SUGAR SC1821 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Short-chain amylose was analyzed using Asahipak NH2P-50 10E (a column for saccharides analysis).
Sample : Amylose
Column : Shodex Asahipak NH2P-50 10E (10mmIDx250mm) Eluent : H2O/CH3CN=45/55 Flow rate : 2.0mL/min Detector : RI Column temp. : 33deg-C
K. Koizumi et al., J. Chromatogr., 585 (1991) 233.
Asahipak ODP-50, a polymer-based reversed phase column, with strong alkaline eluent to prevent anomer separation, oligosaccharides can be separated at room temperature by reversed phase mode. This is an example of one application in which ODP-50 6D enables the separation of oligosaccharides at room temperature with a strong alkaline eluent because polymer-based reversed phase column is resistant to high alkali.
Sample : Amylose
Column : Shodex Asahipak ODP-50 6D (6.0mmID*150mm) Eluent : NaOH aq.(pH10.75) Flow rate : 1.0mL/min Detector : RI Column temp. : 30deg-C
K. Koizumi and T. Utamura, J. Chromatogr., 436 (1988) 328.
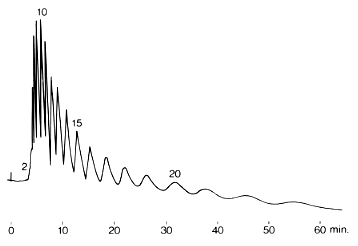
Hydrolyzed starch was analyzed using SUGAR KS-802 (a column for saccharides analysis). Molecular weight distribution of starch syrup whose molecular weight is about 5,000 is measured. Peak tops up to Dp=10 can be observed.
Sample : 1% Hydrolyzed starch, 20micro-L
Column : Shodex SUGAR KS-802 (8.0mmID*300mm) x 2 Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
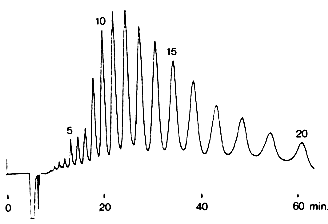
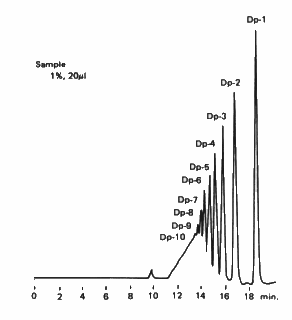
Sweetener was analyzed using SUGAR KS-802 (a column for saccharids analysis). Molecular weight distribution of a sweetener, one of food additives is measured. The majority of the saccharides are less than Dp=6. The largest molecular weight is about 7,000.
Sample : Oligosaccharides
Columns : Shodex SUGAR KS-802 (8.0mmID*300mm) x 4 Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 85deg-C
A mixture of oligosaccharides, sugar alcohols and ethanol is separated using SUGAR SC1211 (a column for saccharides analysis) with the flow rate of 1.0mL/min and 0.5mL/min. The separation can be improved using lower flow rate.
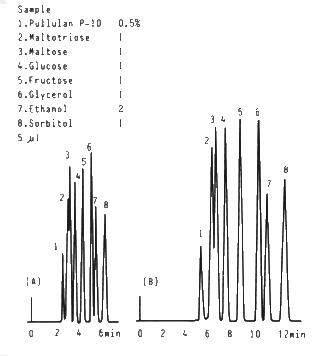
Sample : 5micro-L
1. 0.5% Pullulan P-10
2. 1.0% Maltotriose
3. 1.0% Maltose
4. 1.0% Glucose
5. 1.0% Fructose
6. 1.0% Glycerol
7. 2.0% Ethanol
8. 1.0% Sorbitol
Columns : Shodex SUGAR SC1211 (6.0mmID*250mm) Eluent : H2O Flow rate : (A); 1.0mL/min, (B); 0.5mL/min Detector : Shodex RI Column temp. : 80deg-C
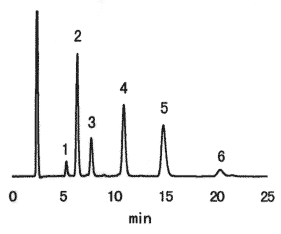
Sample : 2.5% Fructooligosaccharide syrup, 20micro-L
| Sample | Concentration (mg/mL) |
Contents in 100g (g) |
| 1. Fructose | 0.71 | 2.8 |
| 2. Glucose | 5.11 | 20.5 |
| 3. Sucrose | 2.03 | 8.1 |
| 4. 1-Kestose | 5.78 | 23.1 |
| 5. Nystose | 5.91 | 23.7 |
| 6. 1-Fructofuranosyl-D-nystose | 1.95 | 7.8 |
| Total | 86.0 |
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm) Eluent : CH3CN/H2O=70/30 Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 25deg-C
HPLC method is used for the quantitative analysis of low molecular weight dietary fibers. In this analysis, it is necessary to divide the sample into two fractions, one is hard of digestion fraction composed of saccharides equal or larger than trisaccharide (hard of digestion oligosaccharides + dietary fibers) and another is easy of digestion fraction composed of monosaccharides, disaccharides and sugar alcohols. And, the portion of hard of digestion fraction can be considered as the portion of low molecular weight dietary fibers. Analysis of the low molecule water solubility dietary fiber which used SUGAR KS-802 and Asahipak GS-220 HQ was compared. Using GS-220 HQ, monosaccharides, disaccharides and sugar alcohols elute after the position indicated by the arrow mark and it is easy to divide the two fractions. Therefore, the column can be used for the quantitative analysis of low molecular dietary fibers. Using KS-802, the separation of monosaccharides and disaccharides is better.
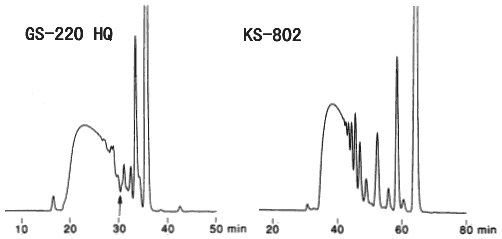
Sample : Dietary fiber
Columns : Shodex Asahipak GS-220 HQ (7.5mmID*300mm) x 2 Shodex SUGAR KS-802 (8.0mmID*300mm) x 2 Eluent : H2O H2O Flow rate : 0.5mL/min 0.3mL/min Detector : Shodex RI Shodex RI Column temp. : 60deg-C 80deg-C
Maltooligosaccharides were analyzed using SB401-4E (a column for high perfonmance semi-micro aqueous SEC (GFC)).
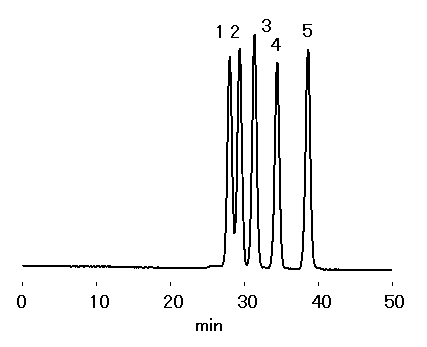
Sample : Maltooligosaccharides, 5micro-L
1. Maltopentaose
2. Maltotetraose
3. Maltotriose
4. Maltose
5. Glucose
Columns : Shodex SB401-4E x 2 (4.6mmID*250mm) Eluent : H2O Flow rate : 0.1mL/min Detector : Shodex RI (small cell volume) Column temp. : 70deg-C
Xylooligosaccharides were analyzed using SUGAR KS-801.
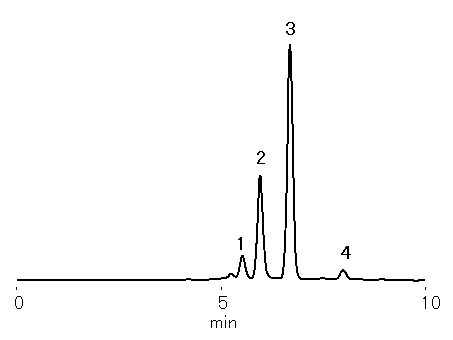
Sample : 0.5% Xylooligosaccharides, 10micro-L
1. Xylotetraose
2. Xylotriose
3. Xylobiose
4. Xylose
Column : Shodex SUGAR KS-801 (8.0mmID*300mm) Eluent : H2O Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 80deg-C
Components of fructooligosaccharide were separated using two sugar columns in series, SUGAR KS-802 and KS-801. Only mobile phase of water was required for this separation.
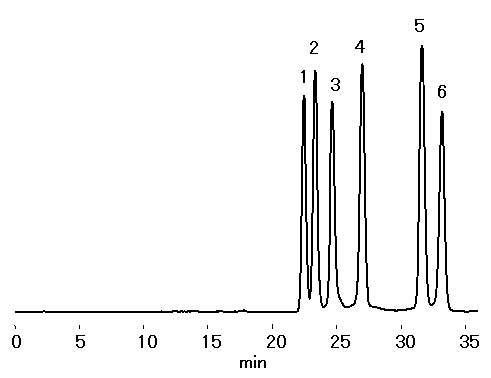
Sample : 0.1% each, 20micro-L
1. 1-Fructofuranosyl-D-nystose
2. Nystose
3. 1-Kestose
4. Sucrose
5. Glucose
6. Fructose
Column : Shodex SUGAR KS-802 + KS-801 (8.0mmID*300mm each) Eluent : H2O Flow rate : 0.5mL/min Detector : Shodex RI Column temp. : 85deg-C
Polymer based amino column, Asahipak NH2P-50 4E was used to analyze cellulose degradation product.
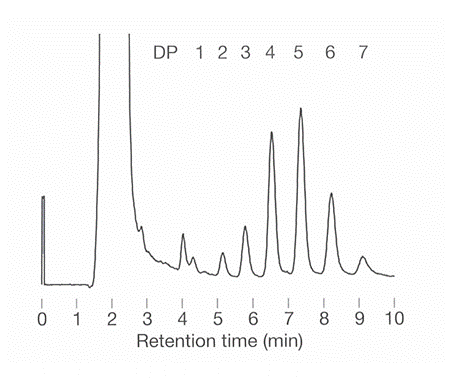
Sample : degradation product of Cellulose
with cellulase digestion
Column : Shodex Asahipak NH2P-50 4E (4.6mmID*250mm)
Eluent : (A); H2O/CH3CN=40/60
(B); H2O/CH3CN=50/50
Linear gradient : (A) to (B), 10min
Flow rate : 1.0mL/min
Detector : Corona charged aerosol
Column temp. : 40deg-C
Data provided by Dr.Kiyohiko Igarashi, Department of Biomaterial Sciences,
Graduate School of Agricultural and Life Sciences, University of Tokyo
Soy bean contains oligosaccharides such as stachyose, raffinose, and sucrose, as well as pinitol and verbascose. Stachyose, raffinose, and verbascose are said to assist increase of bifidobacterium. By using SUGAR KS-801 and KS-802 in series, those oligosaccharides are separated well.
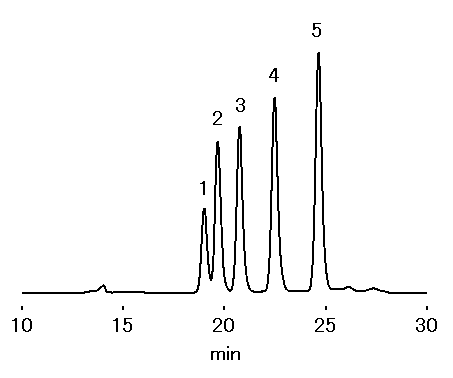
Sample : 0.1% each, 20micro-L
1. Verbascose
2. Stachyose
3. Raffinose
4. Sucrose
5. Pinitol
Column : Shodex SUGAR KS-802 + KS-801 (8.0mmID*300 each) Eluent : H2O Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 85deg-C