Saccharides and Organic Acids
| Saccharides Sugar alcohols |
(a) 5mM |
(b) 20mM |
(b)/(a) |
|---|---|---|---|
| Stachyose | 8.59min | 8.66min | 100.85% |
| Isomaltotriose | 8.85 | 8.90 | 100.59 |
| Panose | 8.94 | 8.98 | 100.48 |
| Maltotriose | 9.03 | 9.09 | 100.65 |
| Gentiobiose | 9.47 | 9.51 | 100.42 |
| Isomaltose | 9.63 | 9.69 | 100.64 |
| Melibiose | 9.77 | 9.82 | 100.51 |
| Kojibiose | 9.79 | 9.83 | 100.44 |
| Maltose | 9.80 | 9.86 | 100.60 |
| Trehalose | 9.82 | 9.87 | 100.53 |
| Nigerose | 9.87 | 9.87 | 99.99 |
| Palatinose | 9.96 | 9.75 | 97.91 |
| Palatinit | 9.96 | 10.04 | 100.77 |
| Trehalulose | 9.97 | 10.02 | 100.51 |
| Lactose | 9.99 | 10.05 | 100.55 |
| Xylobiose | 10.09 | 10.15 | 100.52 |
| Maltitol | 10.19 | 10.27 | 100.78 |
| Lactitol | 10.45 | 10.54 | 100.84 |
| Glucose | 11.32 | 11.37 | 100.47 |
| Sorbose | 11.54 | 11.58 | 100.32 |
| myo-Inositol | 11.69 | 11.75 | 100.47 |
| D-Mannose | 11.85 | 11.90 | 100.44 |
| D(+)-Galactose | 11.89 | 11.94 | 100.44 |
| D(+)-Xylose | 11.92 | 11.97 | 100.43 |
| D(-)-Fructose | 12.01 | 12.05 | 100.37 |
| Mannitol | 12.26 | 12.34 | 100.63 |
| D(-)-Sorbitol | 12.42 | 12.47 | 100.39 |
| D(+)-Rhamnose | 12.46 | 12.51 | 100.41 |
| D(+)-Arabinose | 12.74 | 12.79 | 100.40 |
| D(-)-Ribose | 13.01 | 13.04 | 100.22 |
| D-Arabitol | 13.07 | 13.14 | 100.57 |
| Xylitol | 13.25 | 13.33 | 100.61 |
| D(+)-Fucose | 13.35 | 13.40 | 100.40 |
| meso-Erythritol | 13.81 | 13.89 | 100.60 |
| N-Acetyl-alpha-D- glucosamine |
14.02 | 14.05 | 100.21 |
| N-Acetyl-alpha-D- galactosamine |
15.47 | 15.49 | 100.12 |
| Organic Acids | (a) 5mM |
(b) 20mM |
(b)/(a) |
|---|---|---|---|
| Oxalic acid | 8.46min | 9.38min | 110.90% |
| D-Glucuronic acid | 10.24 | 10.39 | 101.46 |
| Citric acid | 10.25 | 10.48 | 102.26 |
| alpha-Ketoglutaric acid | 10.27 | 11.47 | 111.66 |
| Maleic acid | 10.28 | 11.77 | 114.46 |
| N-Acetylneuramic acid | 10.53 | 10.94 | 103.92 |
| Tartaric acid | 10.67 | 11.06 | 103.65 |
| Pyruvic acid | 11.48 | 12.79 | 111.36 |
| Malic acid | 11.74 | 11.93 | 101.63 |
| Malonic acid | 11.95 | 12.53 | 104.84 |
| trans-Aconitic acid | 12.01 | 12.51 | 104.10 |
| Succinic acid | 13.78 | 13.83 | 100.39 |
| DL-Lactic acid | 14.73 | 14.86 | 100.85 |
| Formic acid | 15.70 | 15.82 | 100.78 |
| Acetic acid | 16.90 | 16.93 | 100.18 |
| Adipic acid | 17.32 | 17.42 | 100.59 |
| Mesaconic acid | 18.28 | 19.02 | 104.07 |
| L-Pyroglutamic acid | 19.00 | 19.53 | 102.77 |
| Propionic acid | 19.45 | 19.54 | 100.40 |
| Alcohol | (a) 5mM |
(b) 20mM |
(b)/(a) |
|---|---|---|---|
| Ethanol | 23.43min | 23.52min | 100.38% |
Column : Shodex SUGAR SH1011 (8.0mmID*300mm) Eluent : 5mM or 20mM H2SO4 aq. Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 60deg-C
With SUGAR SH1011 and SH1821, which operate under ion exclusion and GFC modes, the use of perchloric acid aqueous solution allows analysis of saccharides and organic acids in a single run. By changing the concentration (pH) of the perchloric acid aqueous solution and the column temperature, the elution time of organic acids can be adjusted. However, when using columns such as SH1011 and SH1821 whose counter ions are of H type for the analysis of sucrose in a sample, measurement should be performed at a temperature as low as around 15 deg-C for preventing the decomposition of sucrose.
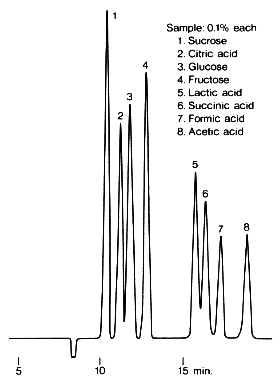
Sample :
1. Sucrose
2. Citric acid
3. Glucose
4. Fructose
5. Lactic acid
6. Succinic acid
7. Formic acid
8. Acetic acid
Column : Shodex SUGAR SH1011 (8.0mmID*300mm) Eluent : 3mM HClO4 aq. Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 15deg-C
Saccharides and sugar alcohols were separated using SUGAR SH1011 (a column for saccharides analysis). In acidic eluent such as sulfuric acid solution, dissociation of organic acids is not so much and they are reteined in the column by reversed phase mode because of the increase of hydrophobicity. However, since organic acids are partly dissociated, minus charge of packing material and plus charge of organic acids repel each other and, therefore, organic acid whose dissociation is lager elutes faster. This mechanism is called as “ion exclusion mode”. Saccharides are eluted by GFC mode.
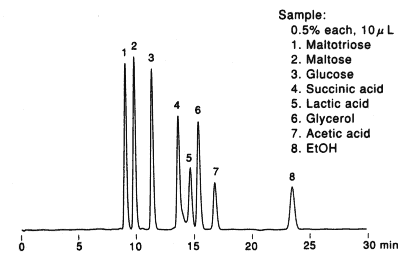
Sample :
1. Maltotriose
2. Maltose
3. Glucose
4. Succinic acid
5. Lactic acid
6. Glycerol
7. Acetic acid
8. Ethanol
Column : Shodex SUGAR SH1011 (8.0mmID*300mm) Eluent : 5mM H2SO4 aq. Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 60deg-C
Lactic acid and Acrylic acid were separated using RSpak DE-413.
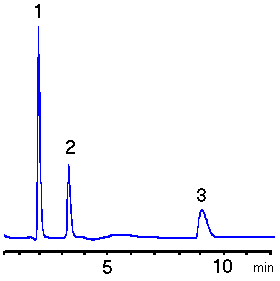
Sample :
1. Glycerol
2. Lactic acid
3. Acrylic acid
Column : Shodex RSpak DE-413 (4.6mmID*150mm) Eluent : 10mM H3PO4 aq. Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 40deg-C
Quantitaive analysis of saccharides and organic acids was performed using SUGAR SH1011. The linearity of the calibration curves are fairly good.
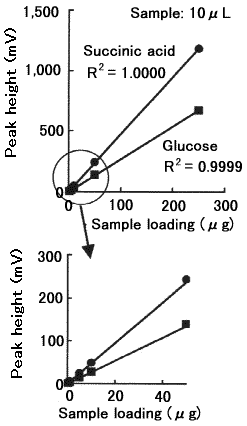
Sample : 10micro-L
|
Correlation Coefficient
(0.2 to 250micro-g) |
|
| Glucose | 0.9999 |
| Succinic acid | 1.0000 |
| Isomaltotriose | 0.9999 |
| Xylitol | 0.9999 |
| Maltitol | 1.0000 |
| Acetic acid | 1.0000 |
| Propionic acid | 1.0000 |
| Adipic acid | 0.9997 |
Column : Shodex SUGAR SH1011 (8.0mmID*300mm)
Eluent : 5mM H2SO4 aq.
Flow rate : 0.6mL/min
Detector : Shodex RI for saccharides
UV(210nm) for organic acids
Column temp. : 60deg-C
With SUGAR SH1011 and SH1821, which operate under ion exclusion and GFC modes, the use of perchloric acid aqueous solution allows analysis of saccharides and organic acids in a single run. By changing the concentration (pH) of the perchloric acid aqueous solution and the column temperature, the elution time of organic acids can be adjusted. However, when using columns such as SH1011 and SH1821 whose counter ions are of H+ type for the analysis of sucrose in a sample, measurement should be performed at a temperature as low as around 15 deg-C for preventing the decomposition of sucrose.
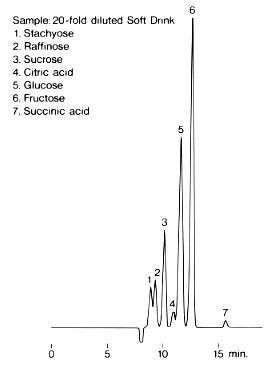
Sample : 20-hold diluted Soft Drink
1. Stachyose
2. Raffinose
3. Sucrose
4. Citric acid
5. Glucose
6. Fructose
7. Succinic acid
Column : Shodex SUGAR SH1011 (8.0mmID*300mm) Eluent : 3mM HClO4 aq. Flow rate : 1.0mL/min Detector : Shodex RI Column temp. : 15deg-C
The results of analysis of samples containing saccharides, organic acids and alcohols using SUGAR SH1821 are shown here. With RI detection, the peaks of these compounds overlap each other. However, with UV detection (at 210nm), there is a possibility of quantifying organic acids as saccharides have little absorbance at 210nm.
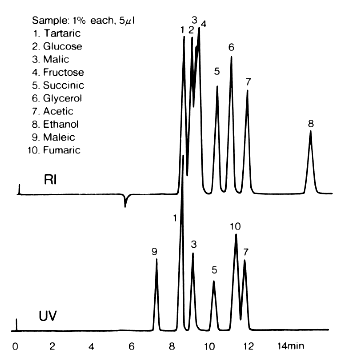
Sample :
1. Tartaric acid
2. Glucose
3. Malic acid
4. Fructose
5. Succinic acid
6. Glycerol
7. Acetic acid
8. Ethanol
9. Maleic acid
10. Fumaric acid
Columns : Shodex SUGAR SH-G (6.0mmID*50mm) + SH1821 (8.0mmID*300mm) Eluent : 5mM H2SO4 aq. Flow rate : 1.0mL/min Detector : Shodex RI, UV(210nm) Column temp. : 50deg-C
SUGAR SH1011 is used for samples of low molecular weight and SUGAR SH1821 packed with material of larger pore size is used for samples containing oligosaccharides. The results of analysis of samples containing saccharides, organic acids and alcohols by SH1011 and SH1811 are shown here. With RI detection, the peaks of these components overlap each other. However, with UV detection (210 nm), there is a possibility of quantifying organic acids as saccharides have little absorbance at 210 nm.
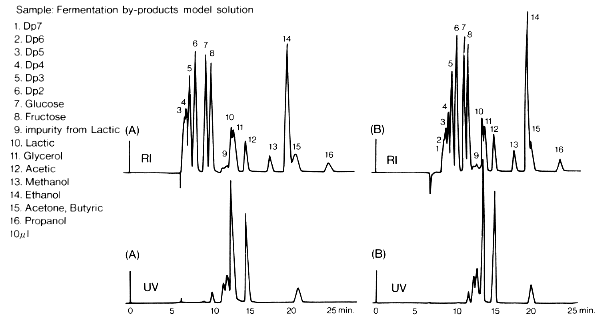
Sample : Fermentation by-products model solution
1. Dp7, 2. Dp6, 3. Dp5, 4. Dp4, 5. Dp3, 6. Dp2,
7. Glucose, 8. Fructose, 9. impurity from Lactic acid, 10. Lactic acid, 11. Glycerol
12. Acetic acid, 13. Methanol, 14. Ethanol, 15. Acetone, Butyric acid, 16. Propanol
Columns : (A) Shodex SUGAR SH-G (6.0mmID*50mm) + SH1011 (8.0mmID*300mm)
(B) Shodex SUGAR SH-G (6.0mmID*50mm) + SH1821 (8.0mmID*300mm)
Eluent : 5mM H2SO4 aq.
Flow rate : 0.75mL/min
Detector : Shodex RI, UV(210nm)
Column temp. : 50deg-C
SUGAR SH1821 column was used to separate malto-oligosaccharides, glucose, lactic acid, glycerol, acetic acid, methanol and ethanol simultaneously in one single run. The separation occurred based on size exclusion, ion exclusion, and reversed phase modes. With the real-time monitoring of fermentation process, appropriate adjustments can be made to maximize the ethanol yield. The retention of organic acids can be controlled by changing the concentration of sulfuric acid in the eluent. It is easy to obtain fast analysis or high resolution data simply by varying the flow rate. The use of a guard column SH-G prolongs the SH1821 column life.
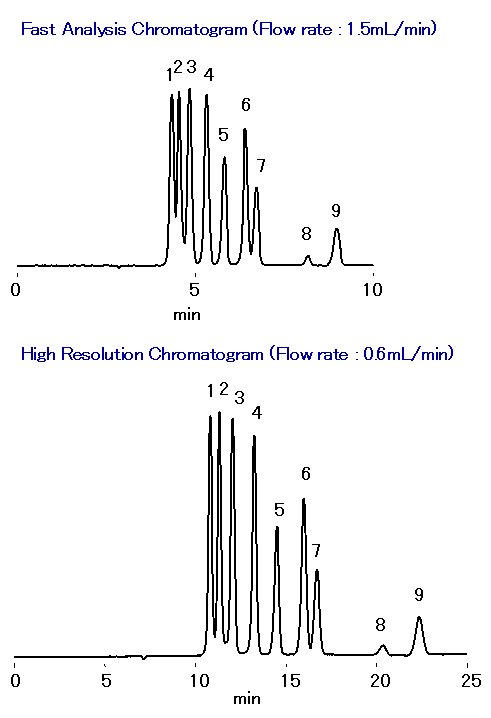
Sample : 0.05% each, 5micro-L
1. Maltotetraose
2. Maltotriose
3. Maltose
4. Glucose
5. Lactic acid
6. Glycerol, Glycerin
7. Acetic acid
8. Methanol, Methyl alcohol
9. Ethanol, Ethyl alcohol
Column : Shodex SUGAR SH1821 (8.0mmID*300mm) Eluent : 0.5mM H2SO4 aq. Flow rate : 1.5mL/min or 0.6mL/min Detector : Shodex RI Column temp. : 75deg-C
In recent years, the use of biomass for bioethanol production has been the object of much research. Depending on the type of biomass and processing method used, furfural or 5-hydroxymethyl furfural (compounds which are known fermentation inhibitors) are produced, In this application the SUGAR SH1821 column was used to simultaneously analyze cello-oligosaccharides along with their constitutive sugars and glucose. SH1821combines ion exclusion, ligand exchange and size exclusion modes and is recommended for the simultaneous analysis of sugars and organic acids.
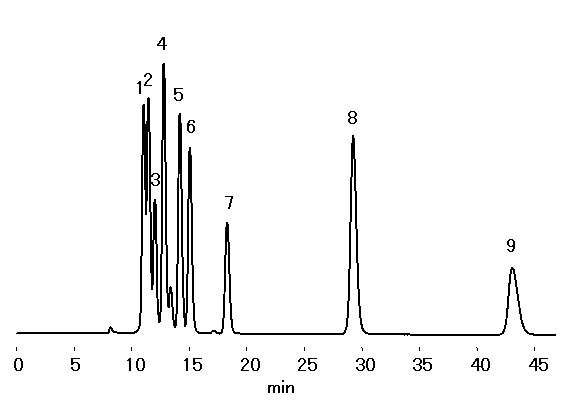
Sample : 0.1% each, 10micro-L
1. Cellopentaose
2. Cellotetraose
3. Cellotriose
4. Cellobiose
5. Glucose
6. Glyceric acid
7. Acetic acid
8. Furfural
9. 5-HMF
Column : Shodex SUGAR SH1821 (8.0mmID*300mm) Eluent : 2mM H2SO4 aq. Flow rate : 0.6mL/min Detector : Shodex RI Column temp. : 60deg-C
